Hbv Dna Iuml
The test can be used to measure HBV DNA levels at baseline and during treatment to aid in.
Hbv dna iuml. Value Flag Reference Range HEPATITIS B DNA QNT PCT 4.0 H < 1.6 log IU **** Viral load result for HBV DNA is 9,450 IU/ML*** 1 IU/ML is 5 copies/mL. The quantitative range of this assay is 1.00-9.00 log IU/mL (10-1,000,000,000 IU/mL). There was no case with persistently normal ALT and HBV DNA levels higher than ,000 IU/mL.
Below the threshold, the viral load is considered “undetectable” – something everyone with chronic hepatitis B wants to hear. A ”Detected” result with the comment, “HBV DNA level is >170,000,000 IU/mL (>8.23 log IU/mL). Liver biochemistries were per-formed in commercially available autoanalyzers.
16.4 IU/mL Quantitative Range:. Table 1 Baseline characteristics of HBV carriers according to the development of abnormal ALT levels or HBV reactivation within one year. Additionally, among patients with HBV DNA higher than 2,000, liver cancer risk increased among those with HBsAg levels higher than 100 IU/mL (OR = 3.72;.
The ULNwas40IU/LforbothALTandAST.HepatitisA,B, D, C virus and HIV 1,2 serological markers (anti-HAV,. A result of "<10 IU/mL (<1.00 log IU/mL)" indicates that HBV DNA is detected, but the HBV DNA level present cannot be quantified accurately below this lower limit of quantification of this assay. - HBV-DNA ≥ 10 5 copies/ml (.000 IU/ml) nếu HBeAg (+) hoặc HBV- DNA ≥ 10 4 copies/ml (2.000 IU/ml) nếu HBeAg (-).
Some patients with CHB have widely fluctuating HBV-DNA levels that may vary from undetectable to >2,000,000 IU/mL. The majority of participants had an ALT <50 IU/L and HBV DNA < 000 IU/ml (59/100). Hepatitis B PCR Interpretation Interpretation.
Hepatitis B Virus DNA, Quantitative, Real-Time PCR - Chronic carriers will persist in producing detectable HBV. Xét nghiệm HBV DNA được theo dõi sau mỗi 3-6 tháng cùng với các xét nghiệm khác (AST, ALT, Creatinine máu, HBeAg, Anti-HBe) để đánh giá đáp ứng. Limitations Assay range is 10 IU/mL to 1,000,000,000 IU/mL.
Care should be taken when interpreting any single viral load determination. Patients with HBV DNA > 000 IU/mL and ALT >2xULN (upper limit of normal), HBV DNA >00 IU/mL and liver stiffness >9 or >12 kPa in case of normal or ≤5xULN, HBV DNA >00 IU/mL and a family history of cirrhosis and/or HCC as well as HBeAg-positive patients with HBV DNA > 000 IU/mL and over 30 years old can begin treatment whatever the liver histology. An "Undetected" result indicates that hepatitis B virus (HBV) DNA was not detected in the serum specimen.
Most HBV-DNA assays used in clinical practice utilize real-time polymerase chain reaction technology with a sensitivity of 5-10 IU/mL and a dynamic range up to 7 log10 IU/mL. This is why a confirmed diagnosis of IC requires quarterly ALT and HBV DNA measurements for at least 1 year, while a single-point detection of combined HBsAg <1000 IU/ml and HBV DNA <00 IU/ml has a robust predictive value for the diagnosis of IC. 1 IU is equivalent to about 5 HBV DNA copies depending on the assay.
These HBV DNA and qHBsAg cutoffs have also been associated with a lower risk for HCC and liver disease progression. HBV DNA IU/mL (Log 10) HBVQNI:. We note at the outset of this review that HBV DNA thresholds may be reported as copies/ml or IU/ml.
ALT and HBV DNA viral loads are shown for the entire cohort in Figure 4. HBV DNA levels between 2,000 and ,000 IU/mL. ≥2 log 10 IU/ml vs.
The HBV DNA level was ≥ 0 IU/mL in 86 (41.3%) patients. HBeAg-Negative Patients with PNALT and HBV DNA 2,000 IU/mL. The conversion factor is 1 IU/ml ≈5.3 copies/ml, but generally a threshold of 00 IU/ml is taken to correspond to 10,000 copies/ml (or 4 log10 copies/ml).
The limit of detection is 12.5 IU/mL. When clinically indicated, follow-up testing with this assay is recommended in 1 to 2 months. Theo dõi trong và sau điều trị:.
The World Health Organization created a standard for measuring HBV DNA, it established the international unit (IU) or copies per milliliter (mL), written as IU/mL or copies/mL. Real-time Polymerase Chain Reaction, Quantitative. My question is what does the 4.0 stand for?.
I had a viral load of Hepatitis C of 0,000 IU/ml for 25 years and only got ill when I was given paracetamol to take daily. HBV DNA检测报告中IU/ml与拷贝/ml间的换算 分类: 肝病检测与检查 | 标签: 乙肝病毒DNA 国际单位 拷贝 换算 17:51 阅读 (7902) 评论 (0). Main inclusion criteria were as follows:.
Results 114 entecavir-treated patients (median age 58.4 years, median serum HBsAg 54.4 IU/mL) with median treatment duration of 6.7 years were recruited. Some Hepatitis B loads are so low as be judged harmless infection and illness wise as they are below 0 to 500, more traces of a past infection than actually having enough Hepatitis B to ever get ill. Hbv dna“< iu/ml”与“tnd”一样吗? hbv dna“< iu/ml”是指hbv dna仍能检测到,只是水平太低,低于 iu/ml这个最低检测值,无法准确读出数值。而“tnd”是在这个标本中根本没有检测到hbv dna。因此,可以认为hbv dna“tnd”这个结果比“< iu/ml”更好。.
HR 2.278, 95%. 95% CI, 1.17-11.) compared with less than. HBV DNA not detected.
The test is most often used to monitor the efficacy of antiviral therapy in individuals with chronic HBV infection. And ≥7 log 10 IU/ml). Is it the same as 9,450 IU/ML?.
HBV DNA test is the best way to approach this group. Cobas ® HBV is an in vitro nucleic acid amplification test for the quantitation of hepatitis B virus DNA in human EDTA plasma or serum of HBV-infected individuals. ASR Analyte Specific Reagent (Analytical and performance characteristics are not established) GPR General Purpose Reagent IVD In Vitro Diagnostics (Rx Only) RUO Research Use Only.
Results exceeding 170,000,000 IU/mL will not be diluted, and will be reported as greater than 170,000,000 IU/mL. A PCR test that quantitates Hepatitis B Virus (HBV) DNA in human plasma or serum from HBV-infected individuals. 1 ng/ml = 0.2 iu/ml or.
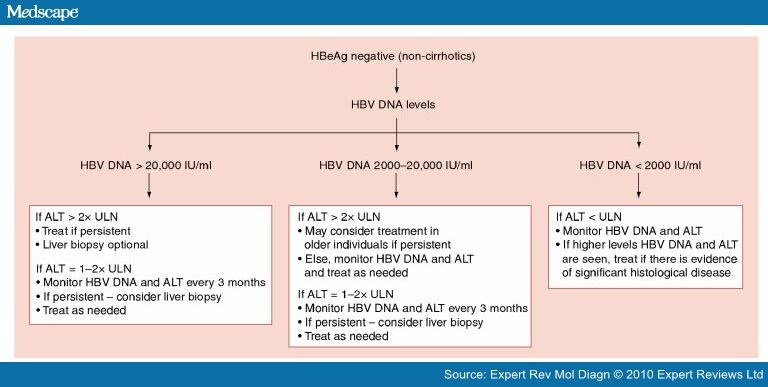
In general, patients with active HBV (HBV DNA ≥ 2,000 IU/mL if HBeAg-negative and HBV DNA ≥ ,000 IU/mL if HBeAg-positive, and high ALT or evidence of advanced fibrosis) should be considered for HBV antiviral treatment. Reference Range See individual components Guidelines. Prophylaxis against HBV reactivation.
1.0E+01 – 1.0E+09 IU/mL (1.0 - 9.0 log IU/mL). The answer above provides general health information that. So i have 47,250 copies/mL?.
Xét nghiệm HBV DNA là một trong những xét nghiệm rất quan trọng và cần thiết.Đối với bệnh nhân bị nhiễm virus viêm gan B thì việc kiểm tra định tính, định lượng 5 hạng mục viêm gan B và đếm số virus viêm gan B là rất cần thiết, tuy nhiên, không phải người bệnh nào cũng có thể hiểu được hết ý nghĩa của. Only treat normal ALT if. The HBeAg status was set as positive or negative, and the baseline HBV DNA load was divided into four grades (<2 log 10 IU/ml;.
Patients between 18 and 65 years of age with hepatitis B surface antigen (HBsAg) positivity for more than 6 months, HBV DNA levels >,000 IU/ml for hepatitis B e antigen (HBeAg)–positive patients or >00 IU/ml for HBeAg-negative patients, and consent for liver biopsy before receiving antiviral therapy. In HBV DNA viral load estimation, what is the equivalent of IU/ml = log10 IU/ml or log10 copies/ml?. The sensitivity of HBV DNA tests may vary with each lab so it’s a good idea to use the same lab for your test.
The optimal management of HBeAg-nega-tive patients with PNALT and HBV DNA 2,000 IU/mL has been the subject of a recent systematic review.9 HBeAg-FIGURE 1. For detection and quantification of hepatitis B virus (HBV) DNA in serum or plasma of patients with confirmed chronic HBV infection to monitor disease progression and response to anti-HBV therapy. HBV DNA is greater than ,000;.
Quantitative measurement of HBV viral DNA may be used to monitor progression of disease. Persons with chronic HBV infection can either be inactive carriers or develop chronic hepatitis. This test measures the actual amount of hepatitis B in a blood sample, which helps determine whether HBV is reproducing in the liver.
The Mean ± SD serum ALT and HBV DNA level was 22.8 ± 8.6 IU/L and 360 ± 4 IU/mL, respectively. If Hepatitis B Virus DNA, Quantitative, Real-Time PCR is ≥600 IU/mL, then Hepatitis B Virus Drug Resistance, Genotype, and BCP/Precore Mutations will be performed at an additional charge (CPT code(s):. So 191.7 x 10 (5) = 19,170,000 copies = 3.5 million IU/mL.
Answer For HBV viral load conversion, 1 IU/ml = 5.6 copies/ml. In a person with detectable HBeAg, an HBV viral load greater than ,000 international units per milliliter (IU/mL) of blood indicates that the virus is active and has the greatest potential to. A positive HBV-DNA level (greater than 115 copies of the virus per mL > IU/mL) indicates that the virus is multiplying in the individual’s body and the person is contagious.
Family history of hepatocellular carcinoma;. Labs usually measure down to less than 0 IU/mL. This test quantitates HBV DNA in serum or plasma with reflex to HBV genotype if ≥500 IU/mL is detected.
This assay cannot accurately quantify HBV DNA above this level” indicates that the HBV DNA level is above the upper limit of quantification for this assay. Determine the number of international units (IU) of hepatitis B virus DNA per milliliter of serum or plasma in known HBV-positive patients. Older age (over 40 years old) Extrahepatic manifestations of HBV;.
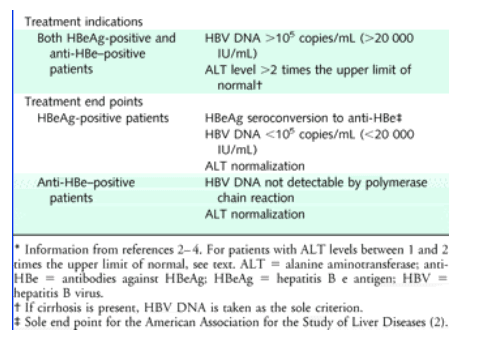
Other factors to consider. Adult over 40 years old with an HBV DNA greater than 1 million IU/mL;. The disease continues to progress in a substantial proportion of patients who achieve standard milestones, such as HBeAg seroconversion, HBsAg clearance, HBV DNA levels less than 105 copies/mL ( 000 IU/mL) or even 104 copies/mL (00 IU/mL), and ALT levels between 0.5 and 2 times the upper limit of normal.
≥4 log 10 IU/ml vs. The 48-week cumulative rate of HBV DNA >00 IU/mL was 58.1%. But not all labs in the United States use this standard.
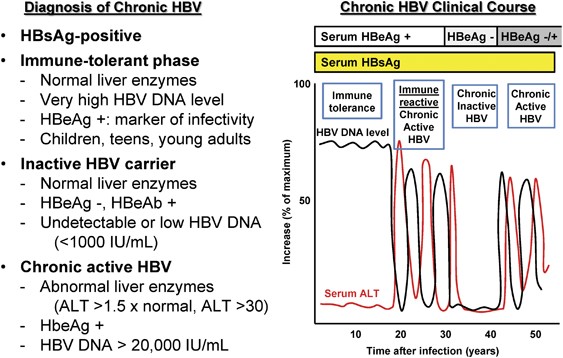
<4 log 10 IU/ml;. A group of patients <30 y of age (circles) had high viral loads and ALTs from 30 to 50 IU/L, representing ‘immune tolerance’. Were converted to IU/mL, considering that 0.15 ng/mL of HBsAg is equivalent to 350 IU/mL." This seems to vary significantly from the usual conversion rules of:.
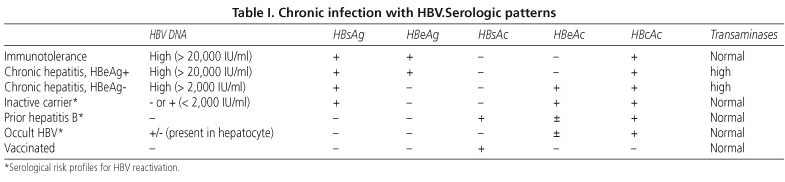
Carefully review criteria before initiating antiviral treatment for HBV. A bi-opsy is recommended before initiating antiviral therapy1,2 (Fig. Inactive carriers refer to HBeAg-negative individuals who have normal serum ALT levels and low (<.
A result of "<10 IU/mL (<1.00 log IU/mL)" indicates that HBV DNA is detected, but the HBV DNA level present cannot be quantified accurately below this lower limit of quantification of this assay. But, the general IU conversion factor is 1.74 copies/IU, or 71,000 IU. This test is intended for use as an aid in the management of patients with chronic HBV infection undergoing anti-viral therapy.
– 170,000,000 IU/mL (1.3 - 9.2 Log IU/mL) 1 IU/mL of HBV DNA is approximately 5. copies/mL A negative result (“Not Detected”) does not rule out the presence of HBV below the limit of detection of the assay or the presence of inhibitory substances. In addition, patients with very low HBsAg (< 100 IU/mL) in combination with an HBV DNA < 00 IU/mL have a high probability of spontaneous HBsAg clearance. End-of-treatment serum HBV RNA and off-treatment serial HBV RNA were both independently associated with HBV DNA >00 IU/mL (HR 2.959, 95% CI 1.776 to 4.926, p<0.001;.
For these patients, HBeAg. The reportable range is – 170,000,000 IU/mL. <7 log 10 IU/ml;.
An interpretation of "Not Detected" does not rule out the presence of inhibitors in the patient specimen or HBV DNA concentration below the level of detection of the test. Patients with chronic liver disease of unknown origin most commonly have HBV that is detected by viral DNA testing. 10 lU/mL - 1.0 x 10 9 IU/mL Performance with HBV DNA-negative samples 100.0% "Target Not Detected" (with a two-sided 95% confidence interval of 99.4% - 100%).
HBV DNA Not Detected <1.00E+1 IU/mL HBV DNA detected below the linear range of the assay. Family history of cirrhosis;.
The Role Of Hbsag Levels In The Current Management Of Chronic Hbv Infection

Comparison Of Different Hepatitis B Guidelines Sethy Pk Goenka Mk Hep B Annual
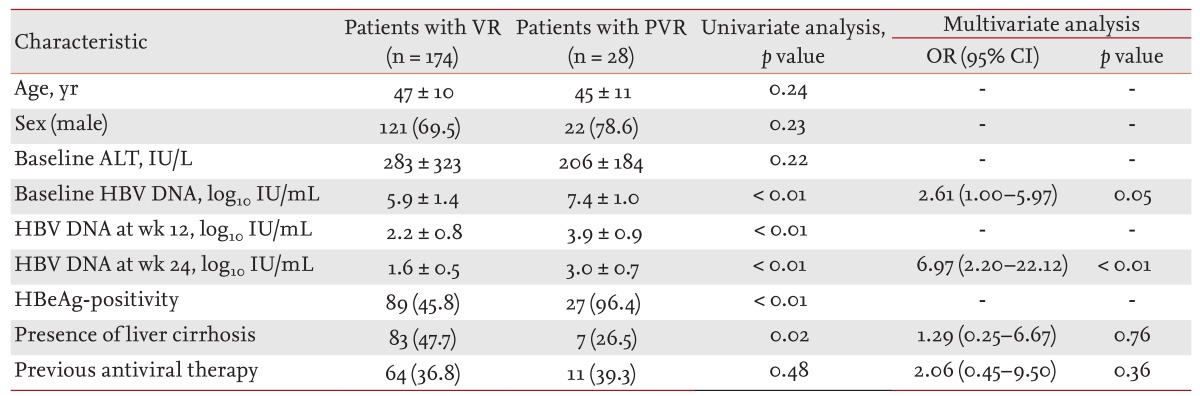
Long Term Virological Outcome In Chronic Hepatitis B Patients With A Partial Virological Response To Entecavir
Hbv Dna Iuml のギャラリー
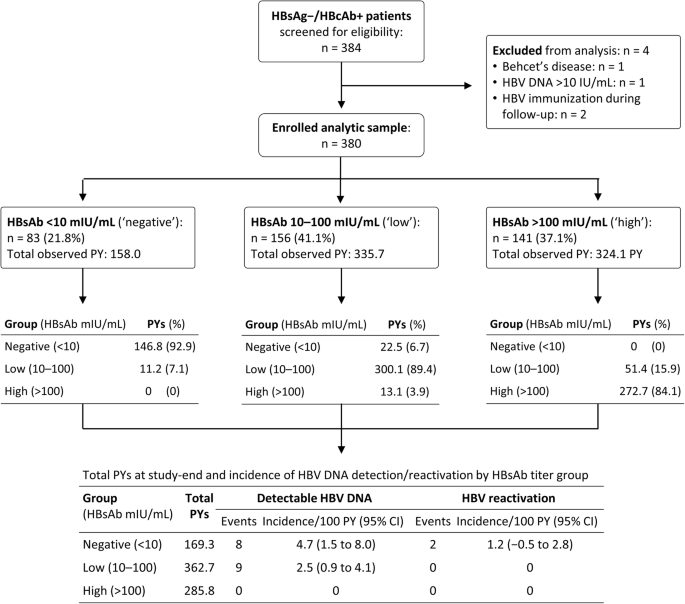
Changes In Hepatitis B Virus Surface Antibody Titer And Risk Of Hepatitis B Reactivation In Hbsag Negative Hbcab Positive Patients Undergoing Biologic Therapy For Rheumatic Diseases A Prospective Cohort Study Arthritis Research Therapy
Plos One Total Hepatitis B Core Antigen Antibody A Quantitative Non Invasive Marker Of Hepatitis B Virus Induced Liver Disease

Chronic Hepatitis B In Pregnant Women Is Hepatitis B Surface Antigen Quantification Useful For Viral Load Prediction International Journal Of Infectious Diseases
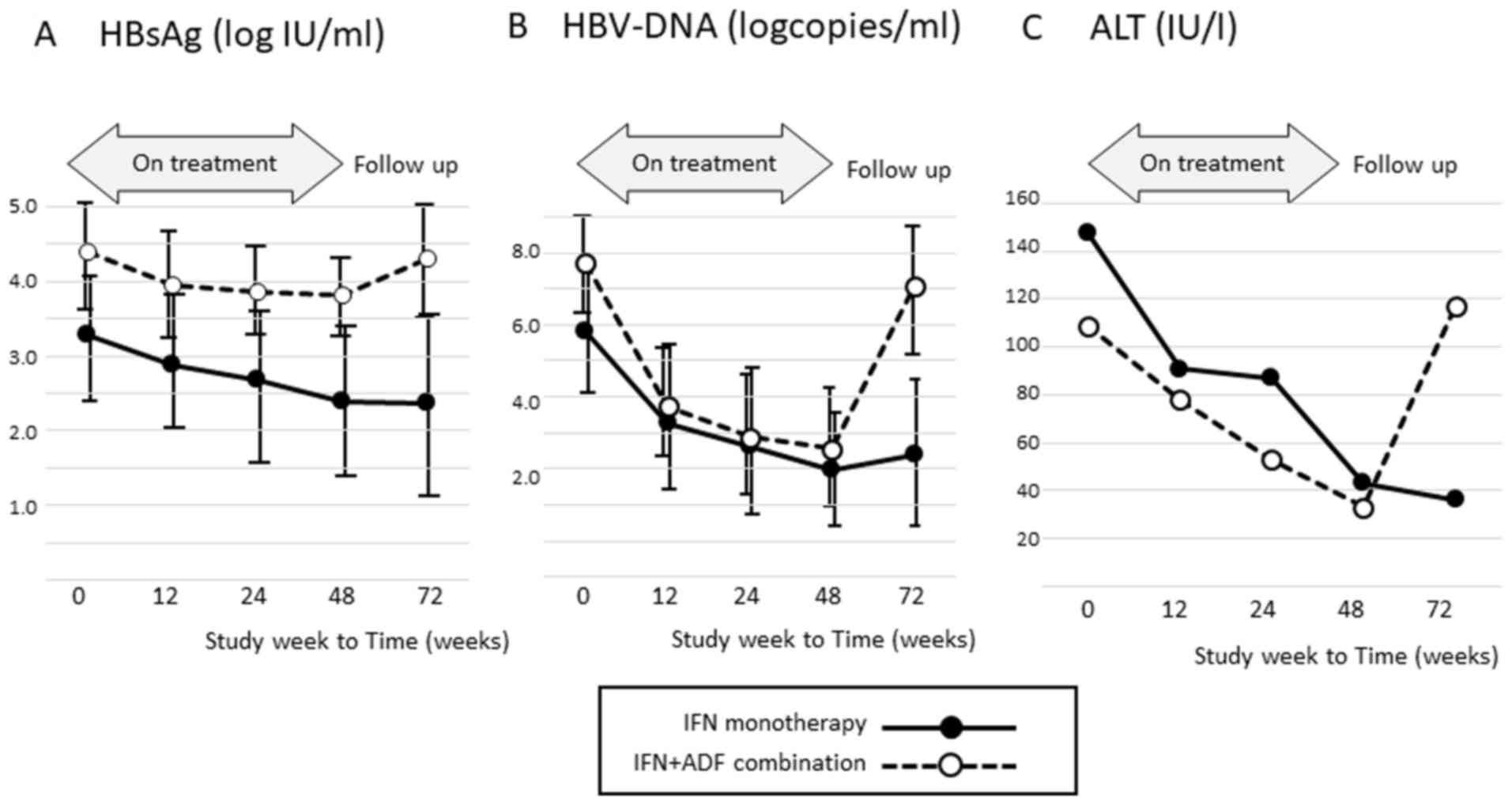
Factors Associated With The Decrease In Hepatitis B Surface Antigen Titers Following Interferon Therapy In Patients With Chronic Hepatitis B Is Interferon And Adefovir Combination Therapy Effective

Association Of Hepatitis B E Antigen And Dna Viral Load With Severity Of Liver Dysfunction And In Hospital Outcomes In Hepatitis B Related Liver Cirrhosis Hou Ame Medical Journal

Association Of Hepatitis B E Antigen And Dna Viral Load With Severity Of Liver Dysfunction And In Hospital Outcomes In Hepatitis B Related Liver Cirrhosis Hou Ame Medical Journal
Q Tbn 3aand9gcqnwp Xvmvowm19jjgu51mzg47ruk0vx S0xbwekonky64drr62 Usqp Cau

Poster Session 3 Hepatitis B Epidemiology And Natural History History 15 Hepatology Wiley Online Library
International Medical Press Browse Articles

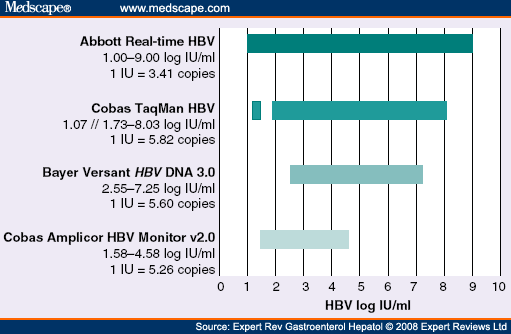
Evaluation Of The Cobas Ampliprep Total Nucleic Acid Isolation Cobas Taqman Hepatitis B Virus Hbv Quantitative Test And Comparison To The Versant Hbv Dna 3 0 Assay Journal Of Clinical Microbiology
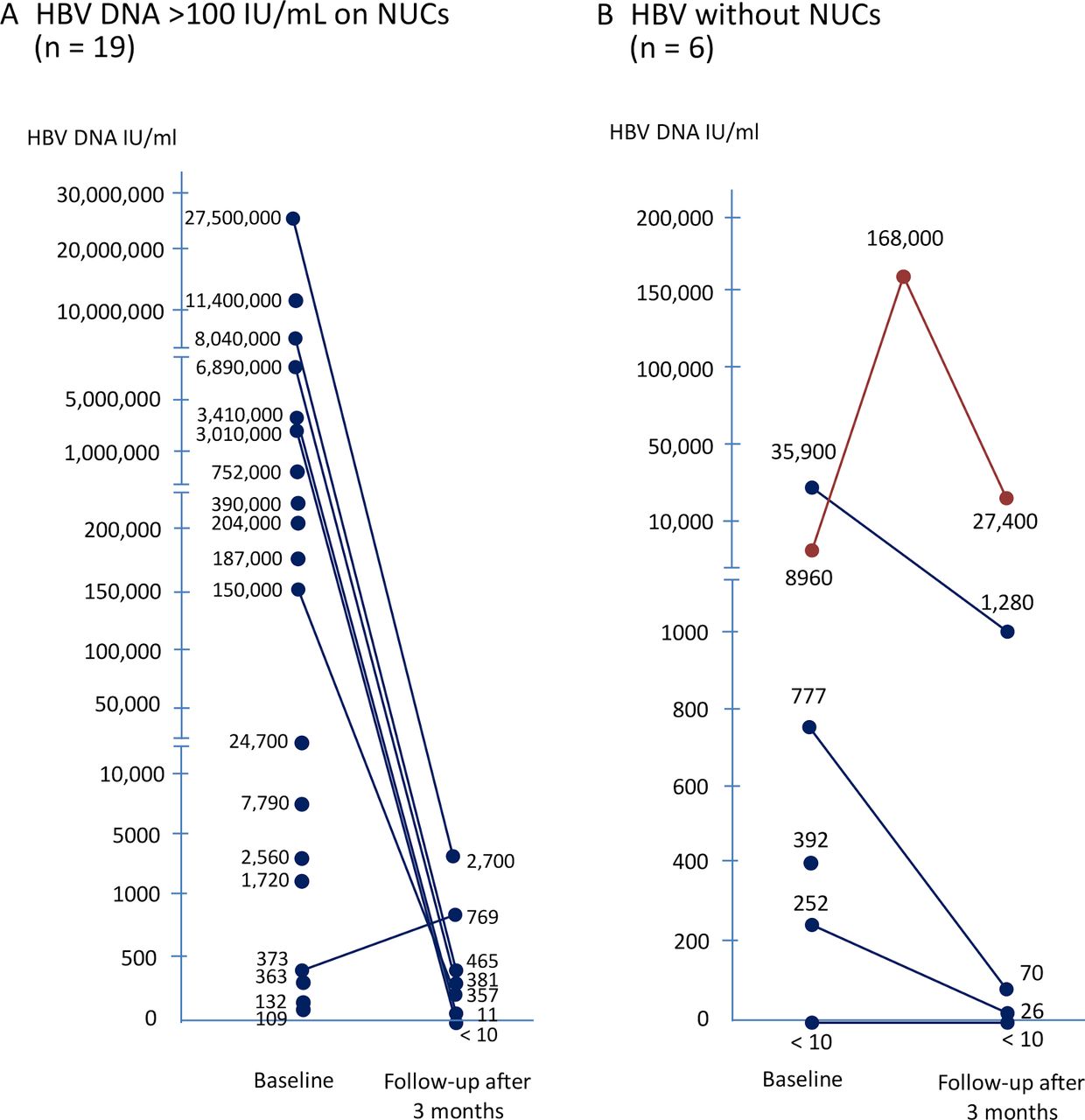
Risk Of Hbv Reactivation In Patients With Immune Checkpoint Inhibitor Treated Unresectable Hepatocellular Carcinoma Journal For Immunotherapy Of Cancer

Serum Hepatitis B Virus Dna Rna And Hbsag Which Correlated Better With Intrahepatic Covalently Closed Circular Dna Before And After Nucleos T Ide Analogue Treatment Journal Of Clinical Microbiology

Serum Hepatitis B Surface Antigen Levels Correlate With High Serum Hbv Dna Levels In Patients With Chronic Hepatitis B A Cross Sectional Study Gupta E Kumar A Choudhary A Kumar M Sarin S K

Hbv Dna Level Of Each Rflp Elisa Hbv Genotyped Group Log Iu Ml Download Table

Fig 36 Coupled Forest Plot Of The Performance Of Hbv Dna 5 Log10 Iu Ml During Pregnancy To Predict Mtct Of Hbv By Type Of Hbv Dna Assay Prevention Of Mother To Child Transmission

Hepatitis B Virus Genotypes Precore Mutations And Basal Core Promoter Mutations In Hbv Infected Chinese Patients With Persistently Normal Alanine Aminotransferase And Low Serum Hbv Dna Levels
Www Cghjournal Org Article S1542 3565 16 1 Pdf
Plos One High Endemicity And Low Molecular Diversity Of Hepatitis B Virus Infections In Pregnant Women In A Rural District Of North Cameroon
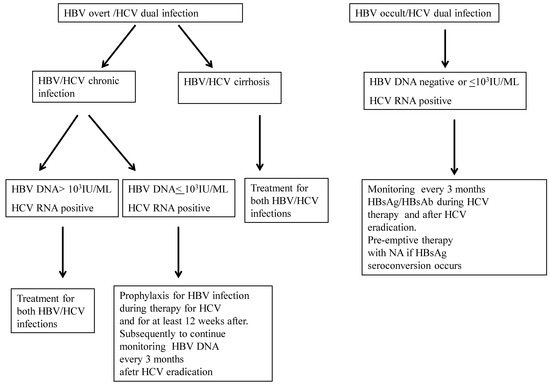
Viruses Free Full Text Hepatitis B Virus Hbv Reactivation Following Pharmacological Eradication Of Hepatitis C Virus Hcv Html
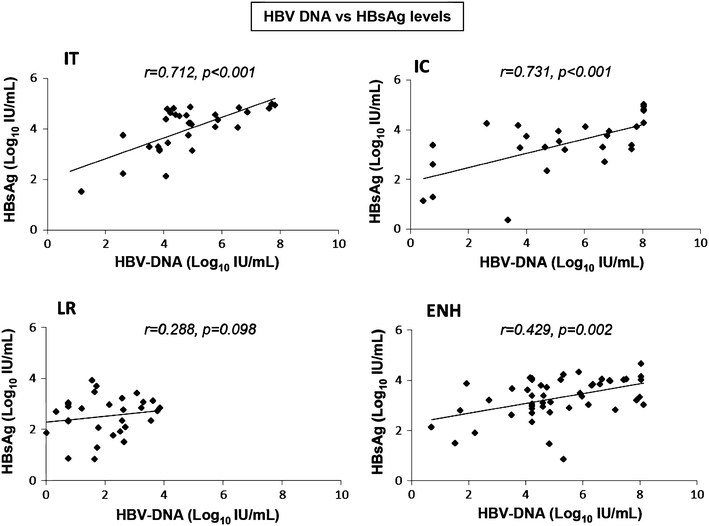
Figure 1 Hbsag Hbeag And Hbv Dna Level Changes And Precore Basal Core Promoter Mutations In The Natural History Of Chronic Hepatitis B In Indonesian Patients Springerlink

Association Between Negative Results From Tests For Hbv Dna And Rna And Durability Of Response After Discontinuation Of Nucles T Ide Analogue Therapy Sciencedirect

Rebound Of Hbv Dna After Cessation Of Nucleos Tide Analogues In Chronic Hepatitis B Patients With Undetectable Covalently Closed Circular Dna Jhep Reports
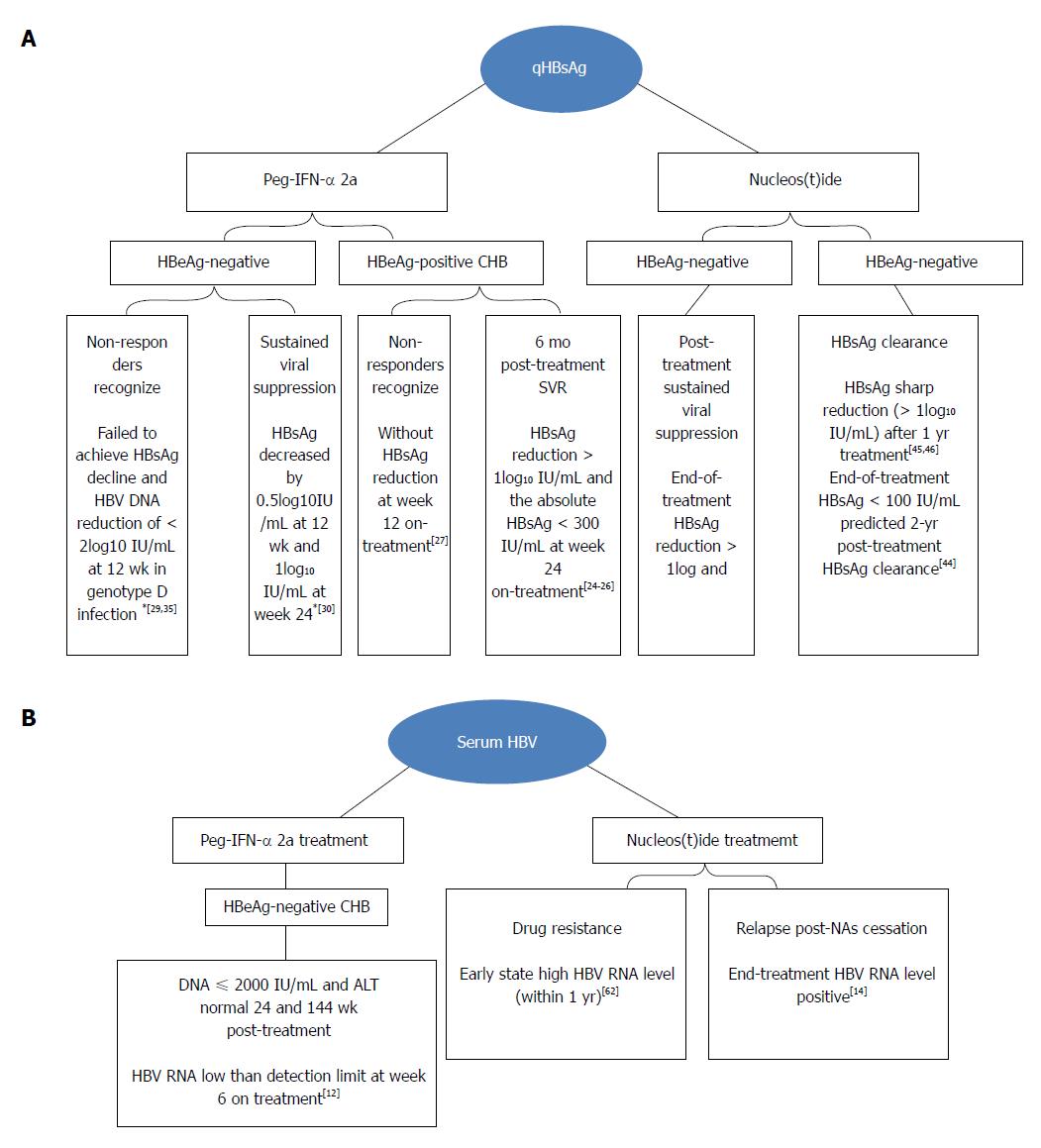
Progression And Status Of Antiviral Monitoring In Patients With Chronic Hepatitis B From Hbsag To Hbv Rna

Zacks Small Cap Research Arwr New Hbv Clinical Data Of Arc 5 Presented At Hep Dart Meeting

Use Of Hepatitis B Virus Core Related Antigen To Evaluate Natural History Of Chronic Hepatitis B Chan Journal Of Gastroenterology And Hepatology Wiley Online Library

Evaluation Of Hepatitis B Virus In Clinical Trials Of Baricitinib In Rheumatoid Arthritis Rmd Open

Performance Comparison Of The Artus Hbv Qs Rgq And The Cap Ctm Hbv V2 0 Assays Regarding Hepatitis B Virus Dna Quantification
Diagnosis Of Hepatitis B Virus Infection

Hepatitis B Virus Dna Stability In Plasma Samples Under Short Term Storage At 42 C
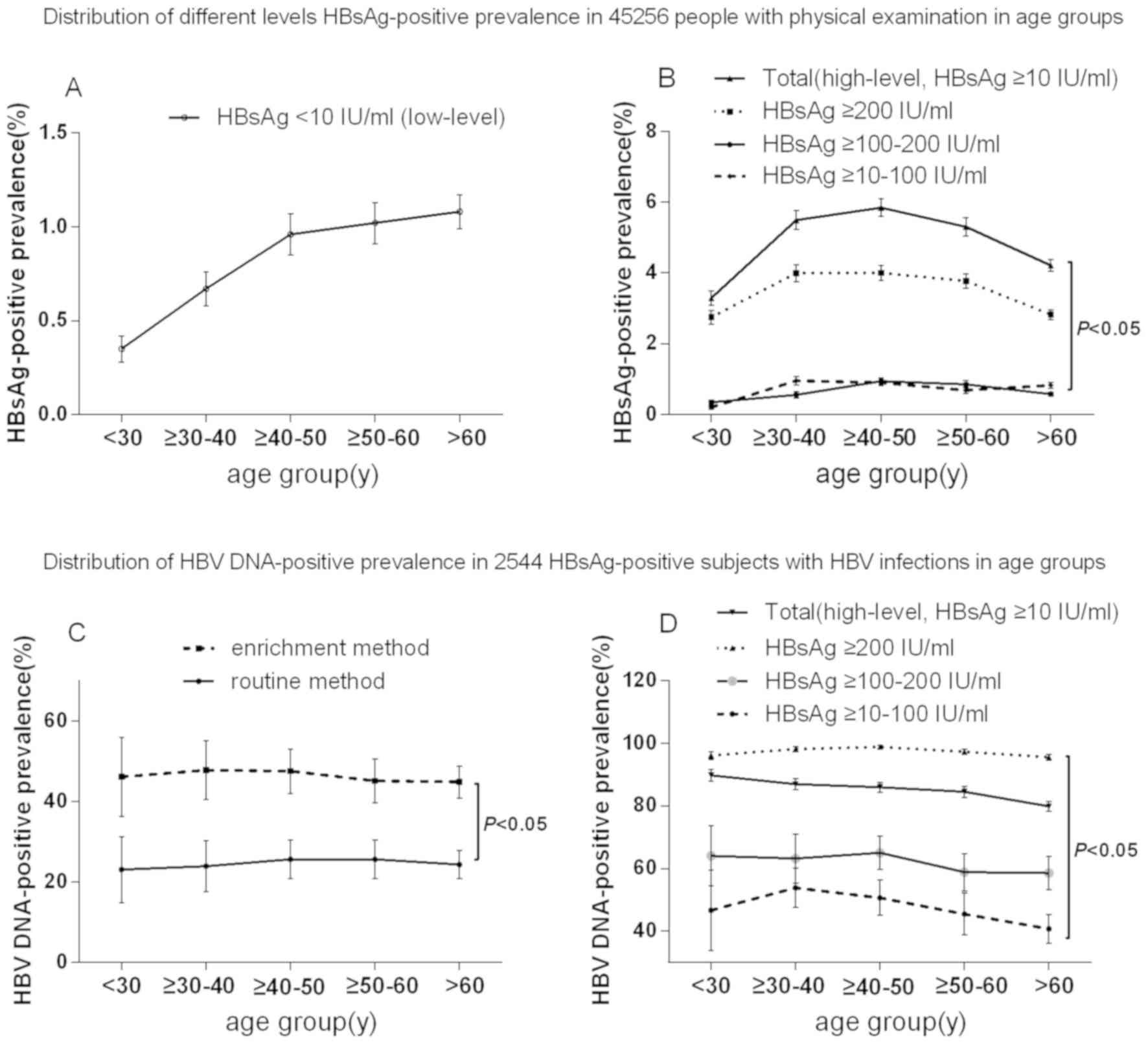
Clinical Characteristics And Association Analysis Of Persistent Low Level Hbsag Expression In A Physical Examination Population With Hbv Infection

Molecular Methods In The Diagnosis And Management Of Chronic Hepatitis B

Hepatitis Monthly Characterization Of Serum Hbv Rna In Patients With Untreated Hbeag Positive And Negative Chronic Hepatitis B Infection

Roc Curve Analysis Using Hbsag 900 Iu Ml For Prediction Of Hbv Download Table
Hepatitis B And C In Pregnancy A Review And Recommendations For Care Journal Of Perinatology
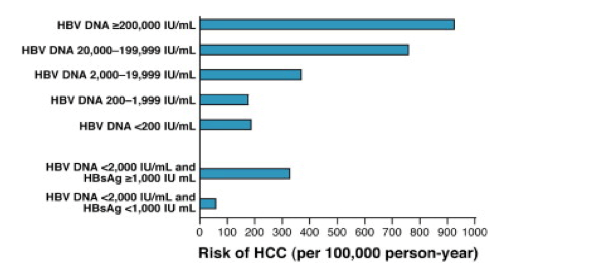
Identifying Hepatitis B Carriers At Low Risk For Hepatocellular Carcinoma Editorial
View Of A Genotype Specific Baseline Score Predicts Post Treatment Response To Peginterferon Alfa 2a In Hepatitis B E Antigen Negative Chronic Hepatitis B Annals Of Gastroenterology

Article
Q Tbn 3aand9gct3oprcobs1fv0fftzrcflzxn1 Aclaov5irr4d3kennel8wc M Usqp Cau

Safety And Efficacy Of Stopping Tenofovir Disoproxil Fumarate In Patients With Chronic Hepatitis B Following At Least 8 Years Of Therapy A Prespecified Follow Up Analysis Of Two Randomised Trials The Lancet

Mean Reduction In Serum Hepatitis B Virus Hbv Dna Log10 Iu Ml From Download Scientific Diagram
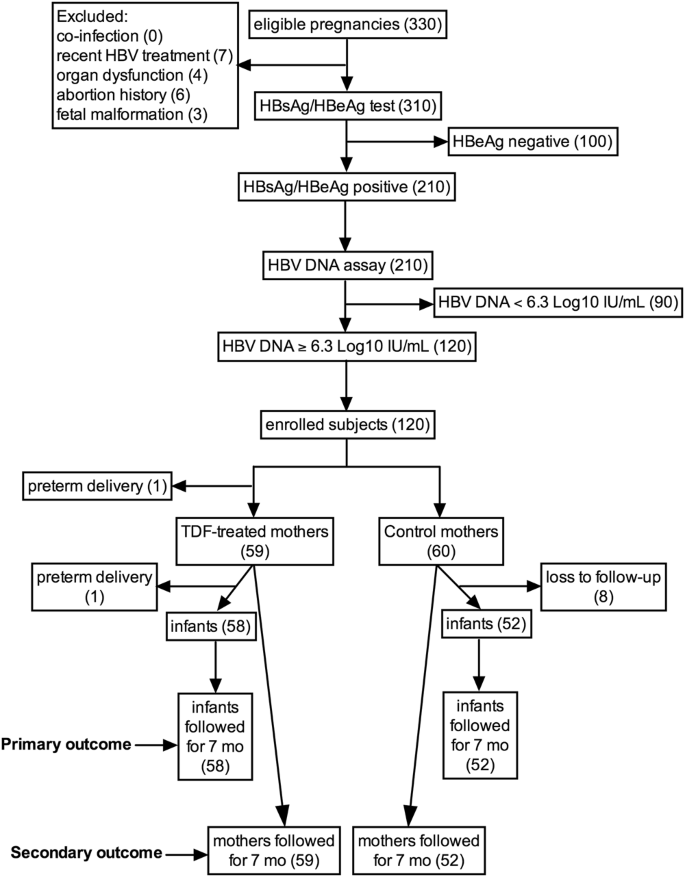
Efficacy Of Tenofovir In Preventing Perinatal Transmission Of Hbv Infection In Pregnant Women With High Viral Loads Scientific Reports
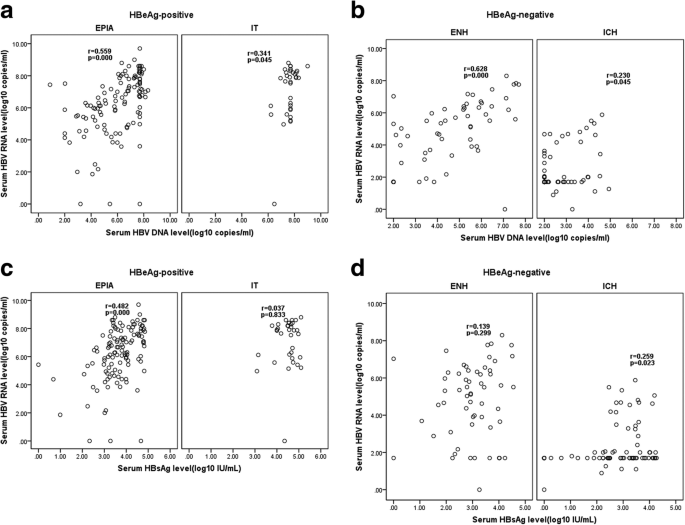
Serum Hbv Rna Quantification Useful For Monitoring Natural History Of Chronic Hepatitis B Infection Bmc Gastroenterology Full Text

Association Between Negative Results From Tests For Hbv Dna And Rna And Durability Of Response After Discontinuation Of Nucles T Ide Analogue Therapy Sciencedirect

Recommendations For Screening Monitoring And Referral Of Pediatric Chronic Hepatitis B American Academy Of Pediatrics

Serum Vitamin D Levels In Treatment Naive Chronic Hepatitis B Patients In Journal Of Translational Internal Medicine Volume 5 Issue 4 17
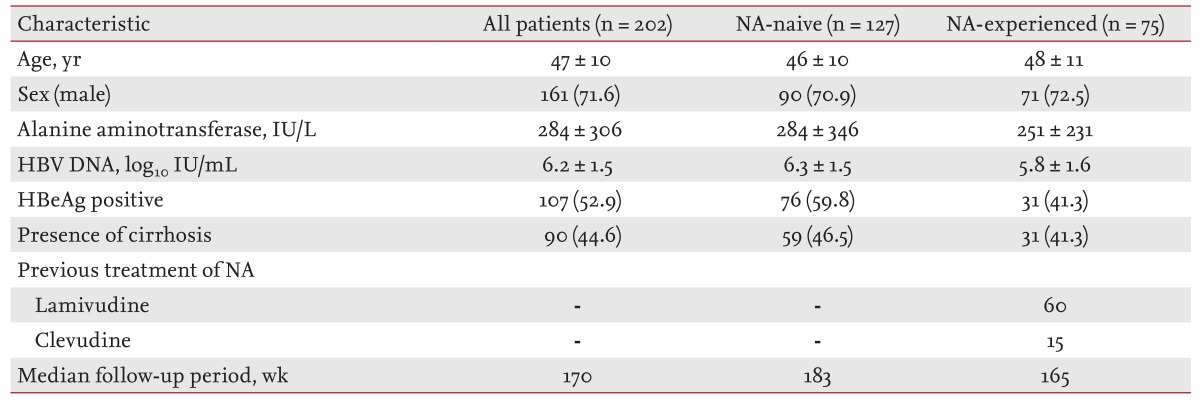
Long Term Virological Outcome In Chronic Hepatitis B Patients With A Partial Virological Response To Entecavir
Www Aphc Info Wp Content Uploads 14 10 Maurizia Brunetto Pdf

Fig 6 Coupled Forest Plot Of The Performance Of Hbeag To Identify Maternal Hbv Dna 5 Log10 Iu Ml By Who Region Prevention Of Mother To Child Transmission Of Hepatitis B Virus Guidelines On
Q Tbn 3aand9gctnczk Zeoncwquzv2jaw2oul3fzjozccljyt0z9wy9fcfvlsjh Usqp Cau
Plos One Prediction Of Clinical Outcomes In Hepatitis B E Antigen Negative Chronic Hepatitis B Patients With Elevated Hepatitis B Virus Dna Levels

Baseline Characteristics Of 251spontaneous Hbeag Seroconverters With Download Table
Hbv Treatment And Management Hepatic Health

Baseline Characteristics Of 251spontaneous Hbeag Seroconverters With Download Table

Inasl Position Statements On Prevention Diagnosis And Management Of Hepatitis B Virus Infection In India The Andaman Statements Journal Of Clinical And Experimental Hepatology

Correlation Between Hepatitis B Surface Antigen Titers And Hbv Dna Levels Alghamdi A Aref N El Hazmi M Al Hamoudi W Alswat K Helmy A Sanai Fm Abdo Saudi J Gastroenterol
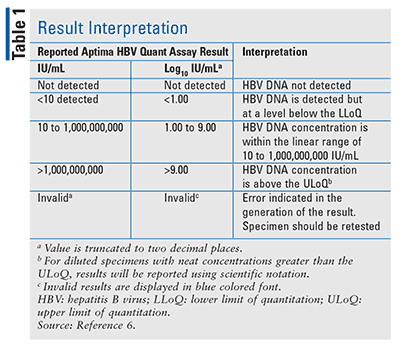
Aptima Hbv Quant Assay

A Real Time Quantitative Assay For Hepatitis B Dna Virus Hbv Developed To Detect All Hbv Genotypes
Prevalence And Characterization Of Occult Hepatitis B Infection Among Blood Donors In Central Saudi Arabia Alshayea Saudi Medical Journal
Reactivacion De La Hepatitis B Y Su Impacto Clinico Actual
Hepatitis B Management In The Pregnant Patient An Update
Q Tbn 3aand9gcrdabd47qhxgwslbvoggjkmkvnkojz1y9nauril5xz93zntykr3 Usqp Cau
Efficacy And Safety Of Tenofovir Disoproxil Furmarate For Patients With Lamivudine Resistant Hepatitis B Virus Infection Kwon Journal Of Gastroenterology And Hepatology Research

Add On Pegylated Interferon Augments Hepatitis B Surface Antigen Clearance Vs Continuous Nucleos T Ide Analog Monotherapy In Chinese Patients With Chronic Hepatitis B And Hepatitis B Surface Antigen 1500 Iu Ml An Observational Study

Chronic Hepatitis B Virus Infection The Lancet

Factors Associated With Active Hbv Replication Hbv Dna 10 Iu Ml Download Table
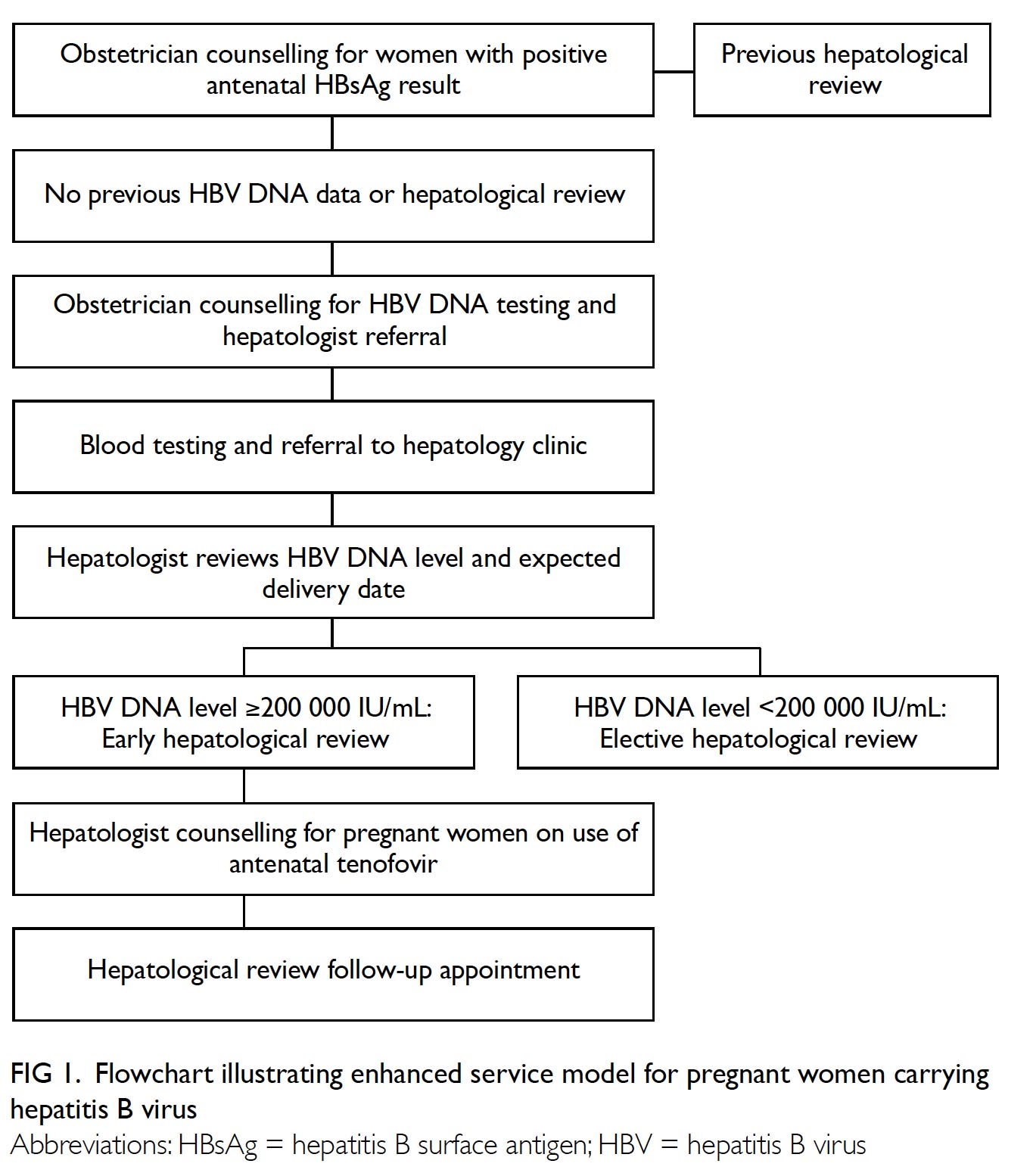
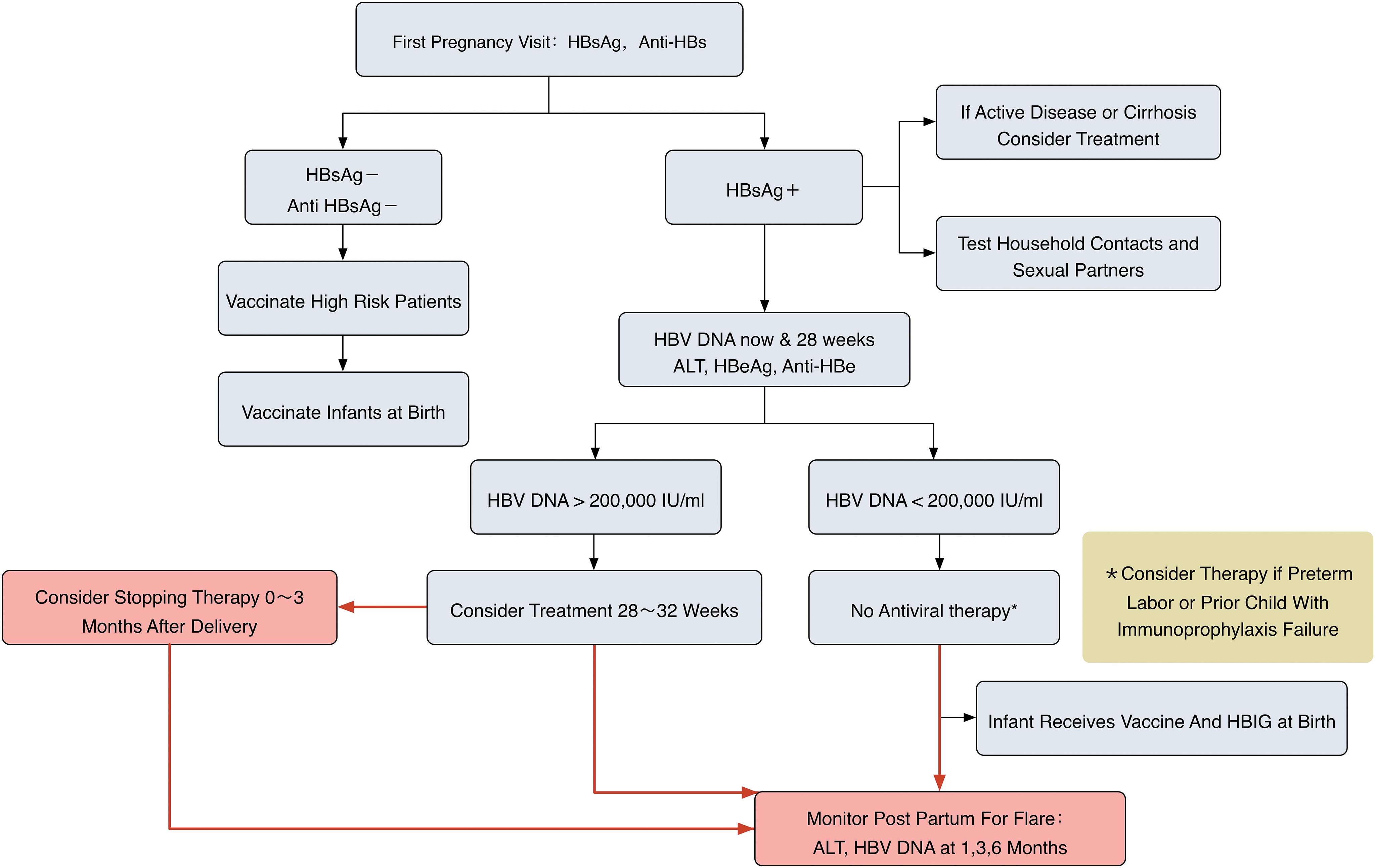
Acceptance Of Antiviral Treatment And Enhanced Service Model For Pregnant Patients Carrying Hepatitis B Hkmj
The Natural History And Treatment Of Chronic Hepatitis B A Critical Evaluation Of Standard Treatment Criteria And End Points Hbv Dna Alt
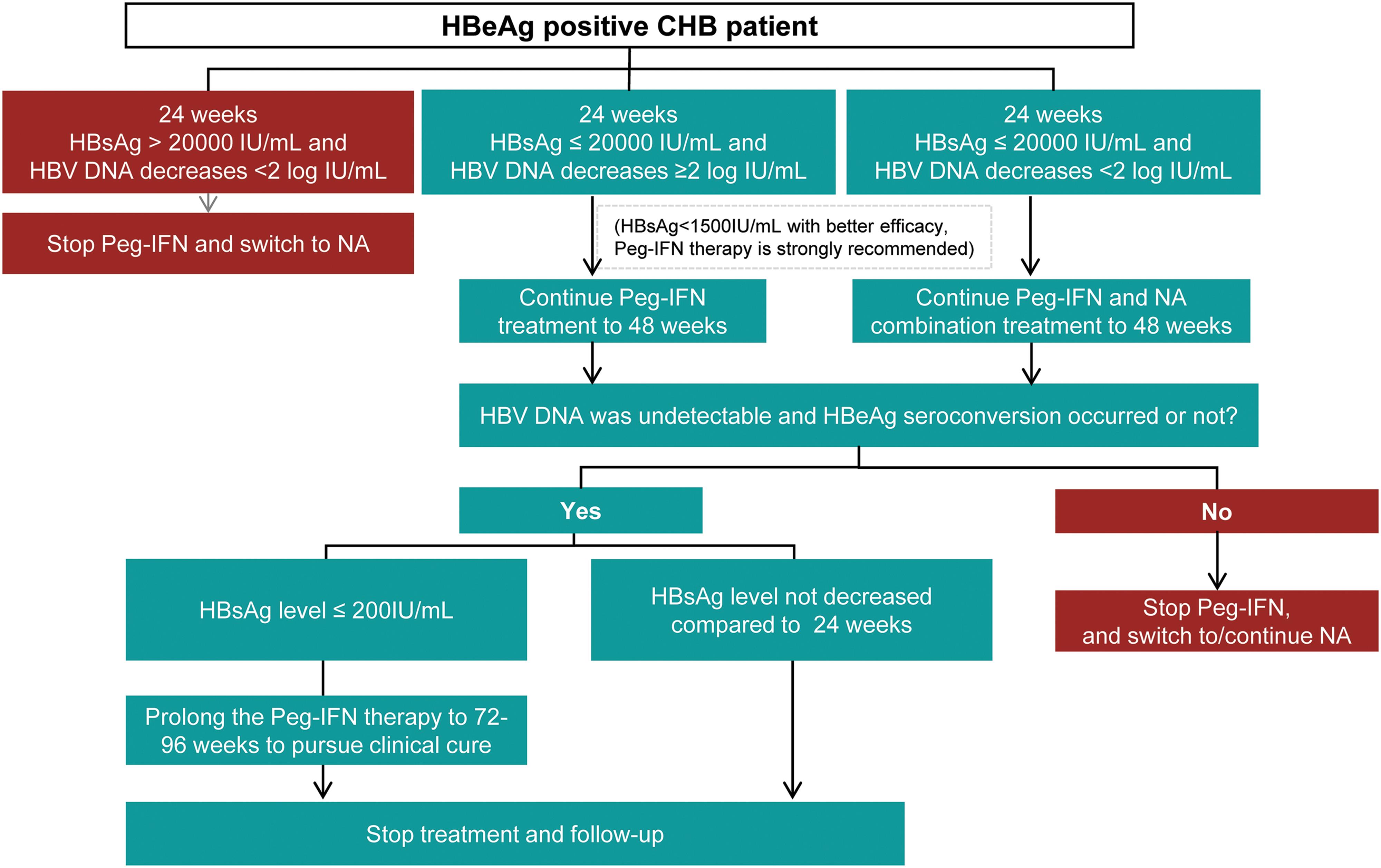
Consensus On Pegylated Interferon Alpha In Treatment Of Chronic Hepatitis B
Www Medicine Uci Edu Gisymposium Pdf Pan Pdf
View Of A Genotype Specific Baseline Score Predicts Post Treatment Response To Peginterferon Alfa 2a In Hepatitis B E Antigen Negative Chronic Hepatitis B Annals Of Gastroenterology
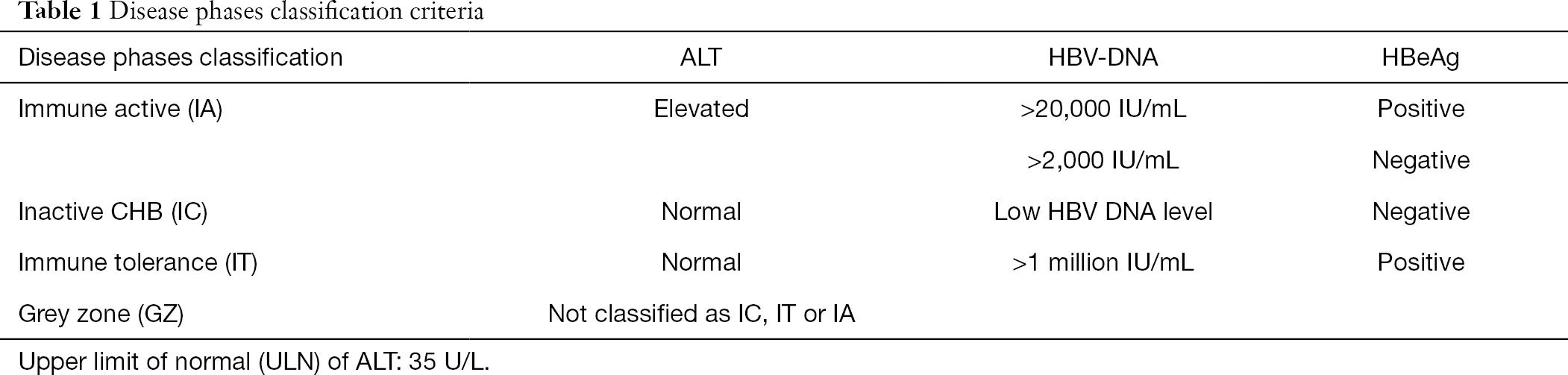
Tim 3 Expression On T Cells Is Correlated With Liver Inflammation Fibrosis And Virological Characteristics In Treatment Naive Chronic Hepatitis B Patients A Cross Sectional Study Gu Annals Of Blood

High Levels Of Hepatitis B Surface Antigen Increase Risk Of Hepatocellular Carcinoma In Patients With Low Hbv Load Gastroenterology

Management Of Hepatitis B In Special Populations Abstract Europe Pmc

Changing Serum Levels Of Quantitative Hepatitis B Surface Antigen And Hepatitis B Virus Dna In Hepatitis B Virus Surface Antigen Carriers A Follow Up Study Of An Elderly Cohort Sciencedirect
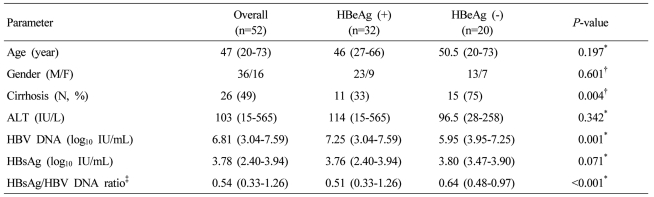
Pretreatment Serum Hbsag To Hbv Dna Ratio Predicts A Virologic Response To Entecavir In Chronic Hepatitis B

Full Text Characteristics Of Hbv Infection In 705 Hiv Infected Patients Under La Idr

Hepatitis Monthly Characterization Of Serum Hbv Rna In Patients With Untreated Hbeag Positive And Negative Chronic Hepatitis B Infection
Www Cghjournal Org Article S1542 3565 19 6 Pdf
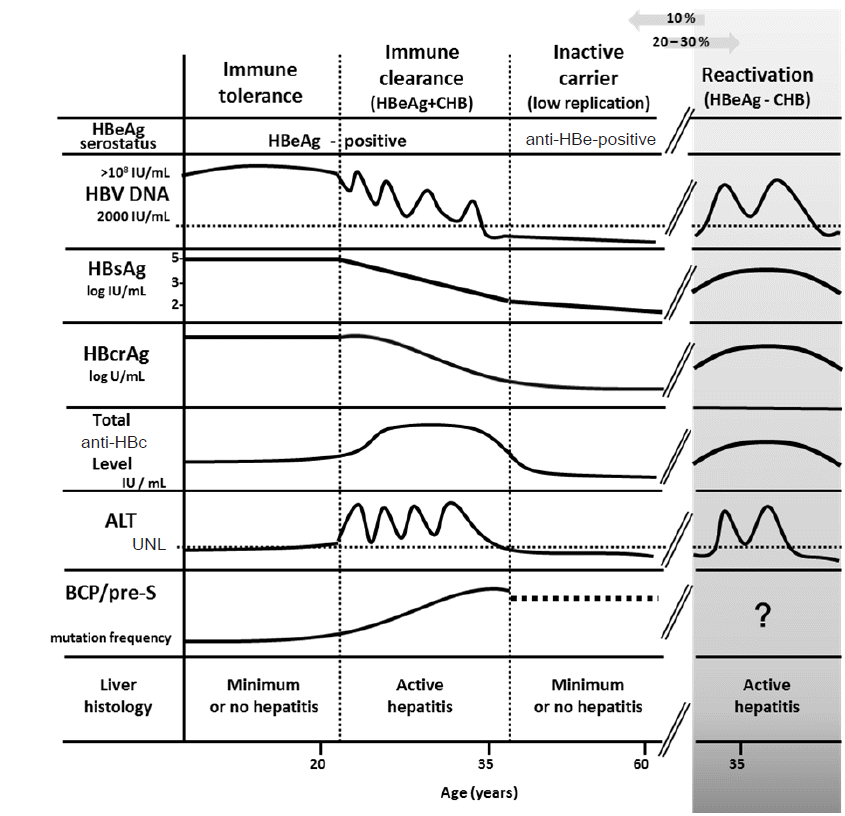
Cmh Clinical And Molecular Hepatology Clin Mol Hepatol 2287 2728 2287 285x The Korean Association For The Study Of The Liver 10 3350 Cmh 16 0069 Cmh 16 0069 Review New Perspectives Of Biomarkers For The Management Of Chronic Hepatitis B

Dominance Of Hepatitis C Virus Hcv Is Associated With Lower Quantitative Hepatitis B Surface Antigen And Higher Serum Interferon G Induced Protein 10 Levels In Hbv Hcv Coinfected Patients Clinical Microbiology And Infection

Management Of Chronic Hepatitis B An Overview Of Practice Guidelines For Primary Care Providers American Board Of Family Medicine

Core Concepts Hepatitis B Co Infection Co Occurring Conditions National Hiv Curriculum
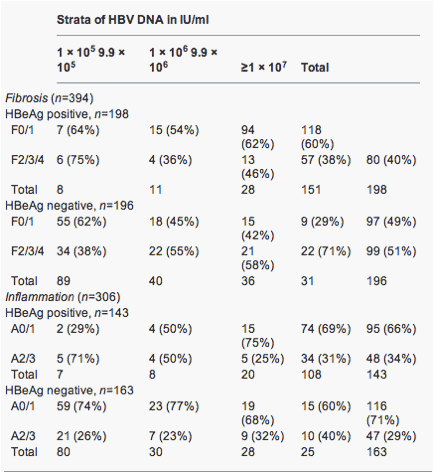
Hbv Viral Load Fibrosis Alt Inflammation Hbeag Hbeag Increasing Hepatitis B Viral Load Is Associated With Risk Of Significant Liver Fibrosis In Hbeag Negative But Not Hbeag Positive Chronic Hepatitis B
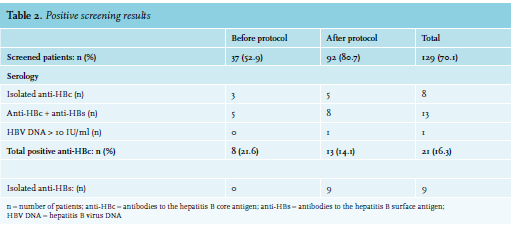
Article Introduction Of Routine Hepatitis B Screening For All Patients Receiving Cancer Treatment Full Text January 19 Njm
Replication Inhibition Of Hepatitis B Virus And Hepatitis C Virus In Co Infected Patients In Chinese Population

Diagnostic Value Of Detection Of Pregenomic Rna In Sera Of Hepatitis B Virus Infected Patients With Different Clinical Outcomes Journal Of Clinical Microbiology
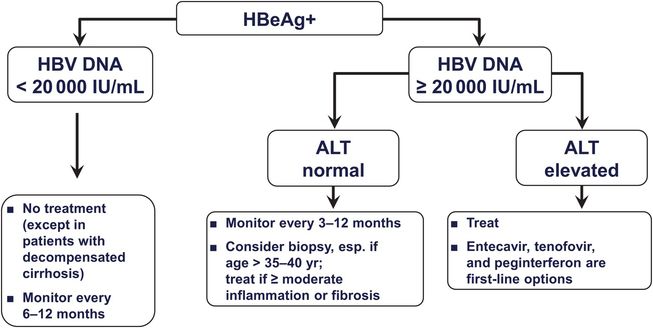
Chronic Hepatitis Oncohema Key

Low Hepatitis B Surface Antigen And Hbv Dna Levels Predict Response To The Addition Of Pegylated Interferon To Entecavir In Hepatitis B E Antigen Positive Chronic Hepatitis B Liem 19
Http Www Gastrojournal Org Article S0016 5085 10 8 Pdf

Serum Hepatitis B Surface Antigen Levels Correlate With High Serum Hbv Dna Levels In Patients With Chronic Hepatitis B A Cross Sectional Study Gupta E Kumar A Choudhary A Kumar M Sarin S K

Performance Comparison Of The Artus Hbv Qs Rgq And The Cap Ctm Hbv V2 0 Assays Regarding Hepatitis B Virus Dna Quantification
Http Regist2 Virology Education Com 17 15euhivhep 13 Battisti Pdf

Full Text Serum Hepatitis B Core Antibody Levels Predict Hbeag Seroconversion In Idr

Mqkae 3nofvuym

Treatment Of Chronic Hepatitis B An Update And Prospect For Cure Intechopen

Comprehensive 18 sld Guidance For Chronic Hepatitis B Gutsandgrowth
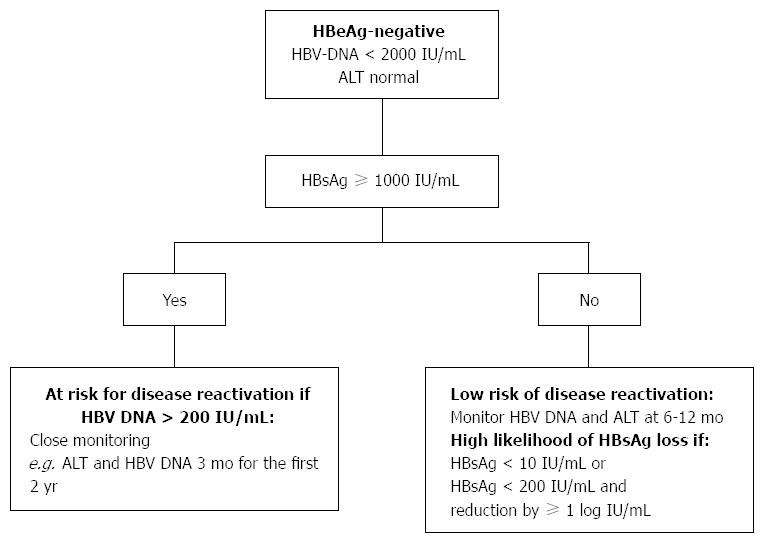
Practical Approach In Hepatitis B E Antigen Negative Individuals To Identify Treatment Candidates

Zacks Small Cap Research Arwr New Hbv Clinical Data Of Arc 5 Presented At Hep Dart Meeting

Characteristics Of Hepatitis B Core Antibody Level In The Natural History Of Chronic Hepatitis B Jian Wang Discovery Medicine
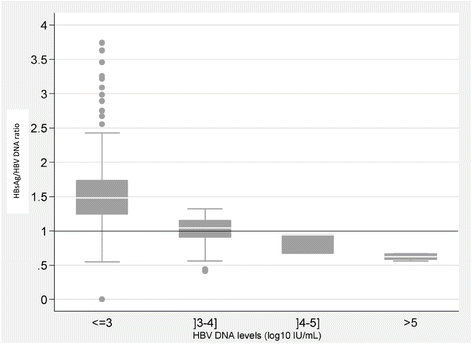
Short Term Spontaneous Fluctuations Of Hbv Dna Levels In A Senegalese Population With Chronic Hepatitis B Bmc Infectious Diseases Full Text
Www Cancerwa Asn Au Resources 18 05 06 Hbv Dr Mitchell Pdf



