Hcv Rna Pcr Qualitative Test Results Normal Range
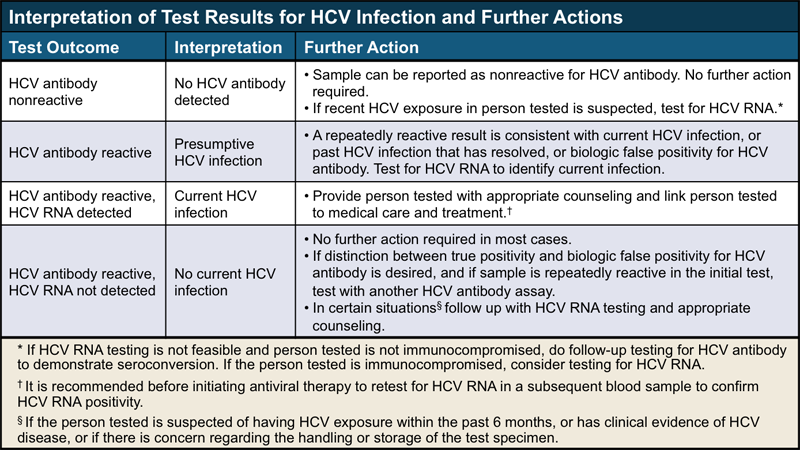
Nucleic acid test (NAT) for HCV RNA positive (including qualitative, quantitative or genotype testing), OR A positive test indicating presence of hepatitis C viral antigen(s) when available* *When and if a test for HCV antigen(s) is approved by the FDA.

Hcv rna pcr qualitative test results normal range. HCV RNA level of below 25 IU/mL in serum or plasma at 12 weeks after ending therapy is the therapeutic goal and indicates an SVR is achieved. Results should be viewed in the context of clinical history and other lab tests. Results can be reviewed directly on the system screen, exported, or printed as a report.
The HCV RNA PCR test is a blood test. If a quantitative HCV RNA test result is reported in a form such as “<615 IU/L,” (under 615) this means that the quantitative test used cannot measure the hepatitis C virus below that number. The result is reported as either "detected" or "not detected." Explanation of test results:.
Hepatitis C RNA PCR Qualitative Blood Test. The performance characteristics of this assay have been determined by North-LIJ Laboratories. Determine the number of international units (IU) of hepatitis C virus (HCV) RNA per milliliter in serum or plasma in known HCV-positive patients Limitations The quantifiable range of the assay is 15 IU/mL to 100,000,000 IU/mL.
A PCR test that offers consolidated HCV viral load and HCV genotype testing. Please consider retesting with a new sample. Normal range for this assay is "Not Detected".
This checks simply if your blood has any HCV. It gives results as positive or negative and, in some cases, has a lower limit of detection below 10 IU/mL. If your results are:.
Hepatitis C virus detection test:. For diagnosis, use the Hepatitis C antibody test which reflexes to HCV RNA qualitative confirmatory test if reactive. For each patient, the result can be described as either a "high" viral load, which is usually >800,000 IU/L, or a "low" viral load, which is usually <800,000 IU/L.
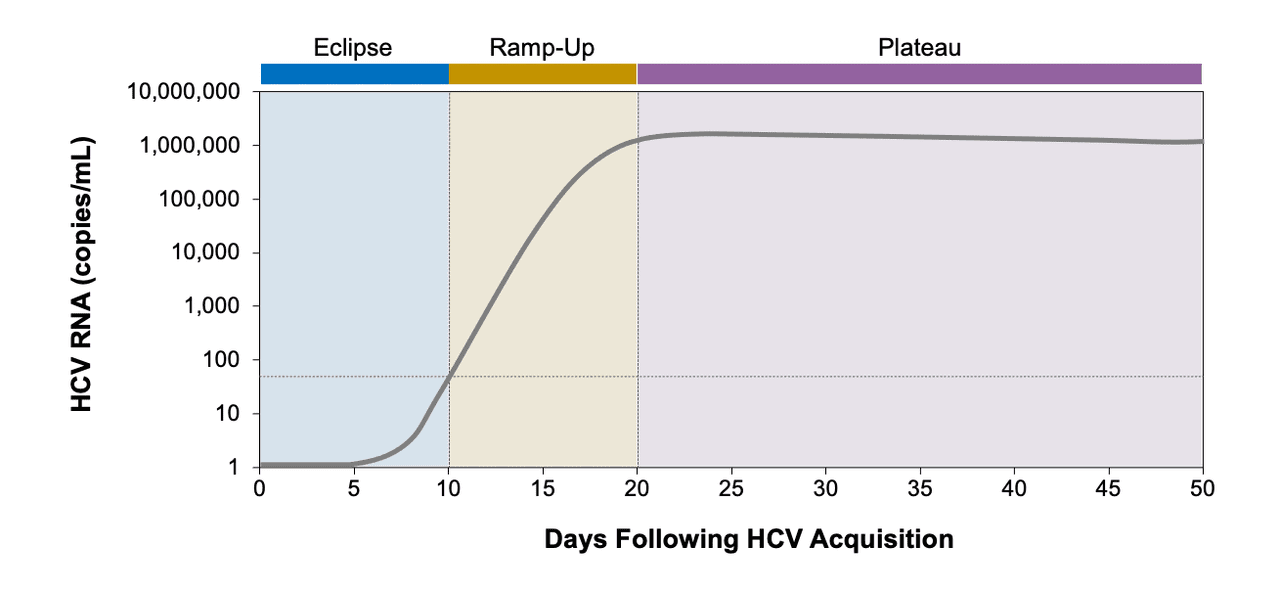
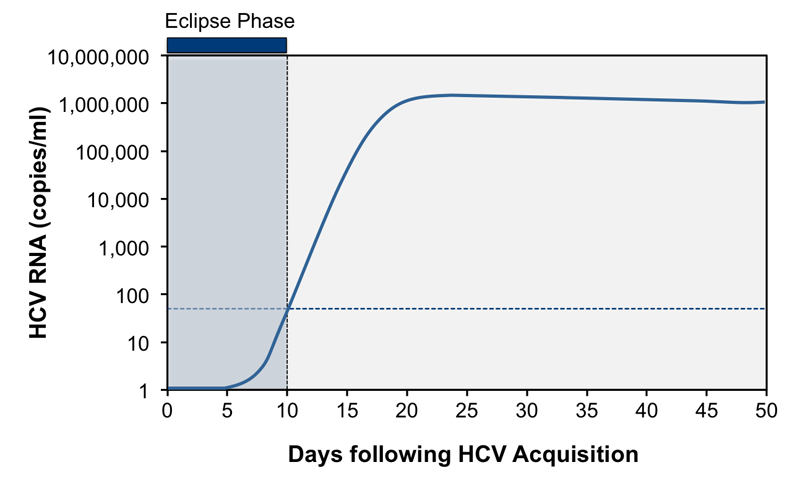
On the other hand, anti-HCV could take about 8-12 weeks before results are positive. Because detection of HCV RNA during the course of infection may be intermittent, a single negative test result for HCV RNA is not conclusive. The Molbio Truenat™ HCV assay (the investigational product) is a quantitative chip-based Real Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR) tests for the detection of HCV genomic RNA from human whole blood, EDTA plasma and serum.
When clinically indicated, follow-up testing with this assay is recommended in 1 to 2 months. 10 IU/mL (1.0 log IU/mL). Hepatitis C specimens for HCV - RNA are tested by the Roche Cobas 6800 Assay System utilizing Real Time PCR (RT-PCR) technology.
Hepatitis C Viral RNA, Quantitative, Real-Time PCR - Useful in monitoring therapy and/or disease progression. In most tests this indicates that HCV has been detected - the test would report “not detected” if no HCV is present - but that the viral load is so low that an accurate value cannot be established. Real-Time Polymerase Chain Reaction (PCR) References:.
Order Code Order Name Result Code Result Name UofM Result LOINC;. Quantitative HBV DNA results generated by this assay may be more than 0.5 log IU/mL lower than those of the VERSANT HBV DNA 3.0 Assay (bDNA) among some clinical serum specimens. Thus, only changes greater than 0.5 log IU/mL from one measurement to the next (or across several measurements) are considered to represent true changes in viral load.
The qualitative HCV RNA tests will report whether the hepatitis C virus is present in the bloodstream or not. This test provides our broadest analytical measurement range. Viral load will be provided if DBS tested has HCV RNA ≥1000 IU/ml.
Hepatitis C Virus (HCV) by Quantitative NAAT with Reflex to HCV Genotype by Sequencing Feedback I want to provide feedback regarding - Select - Test Content or Test Information Pricing and Availability General Usability of Test Directory Look and Feel of Test Directory Request a New Feature in Test Directory. The test has a minimum sensitivity, which is usually reported as “less than 15 IU/ml”. The cobas® 6800/00 software which assigns test results for all tests as target not detected, < LLoQ (lower limit of quantitation), > ULoQ (upper limit of quantitation) or HCV RNA detected, a value in the linear range LLoQ ≤ x ≤ ULoQ.
Qualitative testing is capable of detecting low levels of HCV RNA. A third one, called branched-chain DNA (bDNA), may miss viral. Quantitative HCV RNA testing can be considered at the end of therapy and at 24 weeks or later after completion of antiviral therapy.
This test quantifies HCV RNA of free HCV virions in serum / plasma. Another name used for this test is a PCR test. According to the qualitative HCV RNA results, patients were defined as virologic sustained responders (HCV RNA negative 6 months after the end of therapy) or nonresponders.
• Positive - you now have the virus in your blood. Reportable range is 15 to 100,000,000 IU/mL (1.18-8.00 log IU/mL). But it can be more accurate than the viral load test because it can.
A result of <15 IU/mL (<1.18.log IU/mL) indicates that HCV RNA is detected, but the HCV RNA level present cannot be quantified accurately below this lower limit of quantification of this assay. HCV RNA (International Units) :. A positive test for HCV in the serum indicates active replication of the virus in the liver and possible liver damage.
Normal values for HIV 1 RNA Quantitative PCR Test Price for HIV 1 RNA Quantitative PCR Test Average price range of the test is between Rs.1600 to Rs.8000 depending on the factors of city, quality and availablity. The virus is detected, but the amount can’t be measured. § If the person tested is suspected of having HCV exposure within the past 6 months, or has clinical evidence of HCV disease, or if there is concern regarding the handling or storage of the test specimen.
8 Reporting the viral load results in log IU/mL units helps the healthcare. Roche Diagnostics, Meylan, France). Quantitative testing is typically used before starting treatment to determine a baseline viral load for the purpose of evaluating treatment response.
If a qualitative RNA test is positive (detected), then it is confirmed that the patient has chronic hepatitis C. This test is intended for use as an aid in the management of patients with chronic HCV infection, undergoing antiviral therapy. Therefore, a negative HCV RNA should be followed by a repeat HCV RNA no earlier than six months after a negative HCV RNA test result is obtained.
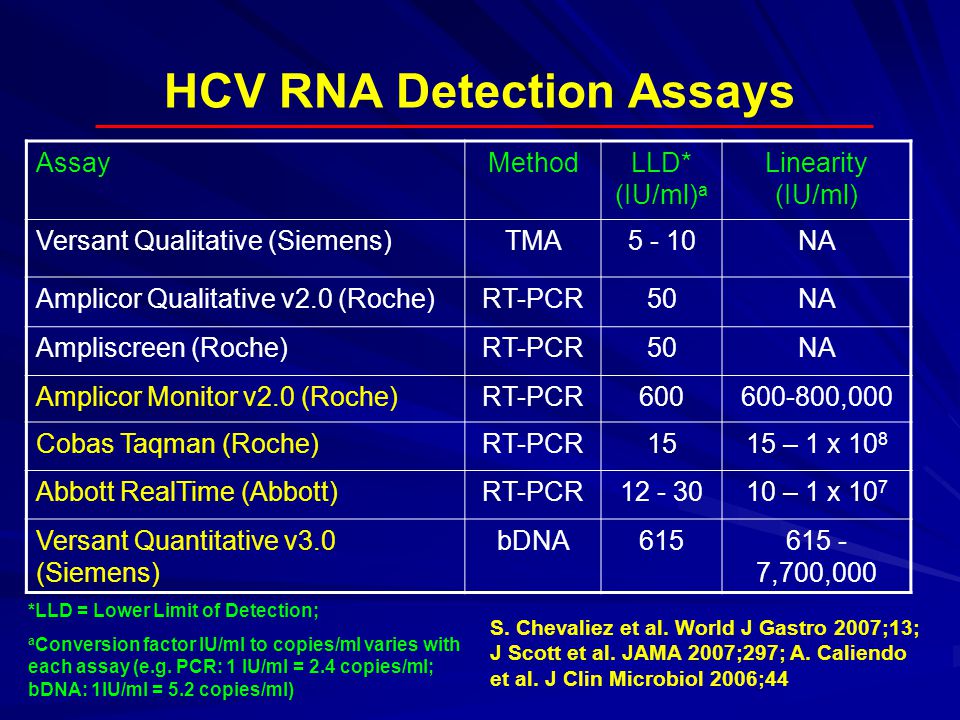
The Truenat™ HCV assay runs on the Truelab™ Uno, Truelab™ Duo or Truelab™ Quadro Dx. There are many different types of RNA technologies, among them the most frequently and predominantly used version is the Polymerase Chain Reaction (PCR) test. The viral load results from the quantitative PCR test can range from 15 to 100,000,000 IU/L.
There is no Hepatitis C normal range in Hepatitis C blood test as the hepatitis test reports are read in terms of positive or negative. SVR, no cirrhosis and normal LFT results (males, ALT ≤ 30 U/L;. The virological response was assessed by a qualitative HCV RNA assay with a lower limit of sensitivity of 50 IU/ml (HCV Amplicor 2.0;.
Both test results are positive in case of acute or chronic infection. † It is recommended before initiating antiviral therapy to retest for HCV RNA in a subsequent blood sample to confirm HCV RNA positivity. Two newer tests -- transcription-mediated amplification (TMA) and polymerase chain reaction (PCR) -- can measure as few as 5-10 IU/mL.
An indeterminate result indicates that HCV RNA result is inconclusive;. The Hepatitis C RNA PCR Qualitative test is used to look for infections with the Hepatitis C virus.This test looks for the genetic material of the virus. My HIV 1 RNA, QN PCR test results reads as follows:.
HCV Real-Time, PCR, Quant:. This test is intended for monitoring viral load during management of HCV infection. Fewer than 15 IU/mL:.
Lower limit of quantitation (LLoQ):. Now available in Dried Blood Spot (DBS). The quantitative range of this assay is 10 - 100,000,000 IU/mL (1.0 - 8.0 log IU/mL).
Females, ALT ≤ 19 U/L):. The COBAS ® AmpliPrep/COBAS ® TaqMan ® HCV Qualitative Test, v2.0 is a qualitative in vitro nucleic acid amplification test for the detection of Hepatitis C Virus (HCV) RNA genotypes 1 to 6 in human EDTA plasma or serum using the COBAS ® AmpliPrep Instrument for automated specimen processing and the COBAS ® TaqMan ® Analyzer or the COBAS ® TaqMan ® 48 Analyzer for automated. People treated with elbasvir plus grazoprevir should have LFTs at Week 8 to screen for hepatotoxicity.
APHL HCV Test Result Interpretation Guide | 1 LABORATORY MARKERS OF HCV INFECTION Currently available Hepatitis C Virus (HCV) antibody tests have a window period from exposure to HCV to detection of antibody of approximately 8-11 weeks. 3.2 H, Reference range:. If the HCV Quantitative PCR result is greater than or equal to 179 IU/mL (2.3 log IU/mL), the HCV Genotype by PCR and Line Probe assay will be added.
You can also get a different kind of RNA test, called a “qualitative” test. After an acute HCV infection, HCV RNA could be detectable in serum within 2 weeks following exposure. LFTs, HCV PCR (qualitative) More intensive monitoring may be required in certain populations (see text).
Although this quantitative HIV-1 RNA test is not FDA-approved for diagnostic purposes, the U.S. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Abbott M00 Note:. Results The PoC assay identified all major HCV genotypes, with a limit of detection of 2362 IU/mL (95% CI 1966 to 27).
Using 422 patients chronically infected with HCV and 503 controls negative for anti-HCV and HCV RNA, the Genedrive HCV assay showed 98.6% sensitivity (95% CI 96.9% to 99.5%) and 100% specificity (95% CI 99.3% to 100%) to detect HCV. Current guidelines for the management and treatment of HCV recommend quantitative testing for HCV RNA before the start of antiviral therapy, during therapy and approximately two months following the end of treatment. Because viral genetic material may be detectable earlier than antibodies which develop in response to an infection, PCR testing can be used to screen for a recent exposure.
1572 H, Reference range:. If the NAT for HCV RNA is:. The 4.8 log 10 dynamic range of the TaqMan HCV quantitative test was comparable to that of the other homogeneous format tests, which were linear over a 5 log 10 23, 26 and 7 log 10 25 range;.
Viral load will be provided if sample tested has HCV RNA ≥15 IU/ml. Polymerase chain reaction is a lab method that takes small amounts of RNA and amplifies it. The quantitative HCV RNA test is checked before a patient starts treatment.
The COBAS ® AmpliPrep/COBAS ® TaqMan ® HCV Qualitative Test, v2.0 is a qualitative in vitro nucleic acid amplification test for the detection of Hepatitis C Virus (HCV) RNA genotypes 1 to 6 in human EDTA plasma or serum using the COBAS ® AmpliPrep Instrument for automated specimen processing and the COBAS ® TaqMan ® Analyser or the COBAS ® TaqMan ® 48 Analyser for automated. Reflex Table for HCV RNA NAA Qualitative;. <1.3 Log copies/mL What in the world does this mean?.
This assay is used to determine the HCV Genotype to aid in prognosis and treatment selection. The HCV RNA test is used to confirm positive HCV serological tests and to demonstrate the presence of HCV RNA in the blood. Replicating PCR test results using the same specimen can vary analytically by as much as 0.5 log IU/mL;.
It may mean that there is no detectable HCV RNA in the patient at all, but it may also mean that the level of virus is just too low for the test to pick it up. A lab technician looks for the genetic material of the HCV virus, or its ribonucleic acid (RNA). This is the most sensitive HCV RNA test available.
24 It is possible that the TaqMan HCV quantitative test would have exhibited an even broader dynamic. PCR makes quantifying the amount of hepatitis C RNA in the blood easier to do. Department of Health and Human Services Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children recommends the use of molecular-based.
This HCV RNA test uses a process known as real-time polymerase chain reaction. By combining real-time PCR and transcription mediated amplification (TMA) technology into a progressive test, we can report a viral load range between 5 and 69,000,000 IU/mL. RNA testing is a cutting edge technology which is used to detect ribonucleic acid from the Hepatitis C virus (also called HCV).
Linearity was not evaluated for one of the assays. They use a process called a polymerase chain reaction (PCR). Nucleic acid amplification tests (NAT) can detect HCV RNA approximately 1-2 weeks after exposure.
This test is not licensed by the FDA as a screening test for HIV-1 infection in donors of blood, human cells, tissues, or tissue products. HCV RNA is a marker of viral replication and persistence is. How can we help?.
This test is intended for use as an aid in management of HCV infected patients and is not intended for use in the initial diagnosis or confirmation of HCV infection. This test is called a nucleic acid test (NAT) for HCV RNA. Mayo Medical Laboratories December 14.
Usefulness Of A Fourth Generation Elisa Assay For The Reliable Identification Of Hcv Infection In Hiv Positive Adults From Gabon Central Africa
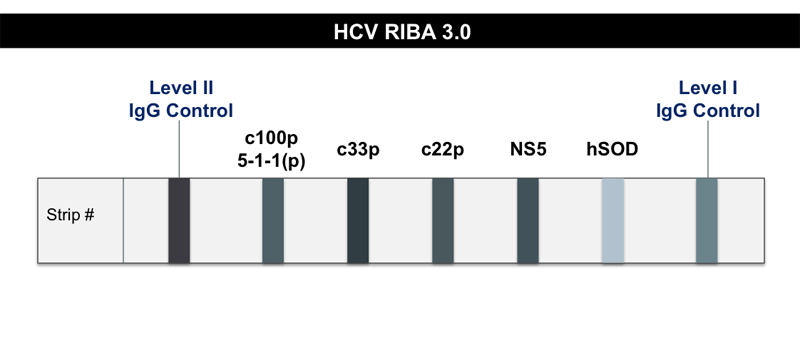
Core Concepts Hepatitis C Diagnostic Testing Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online
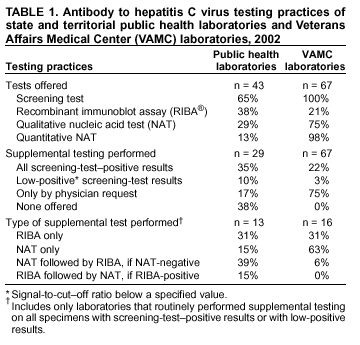
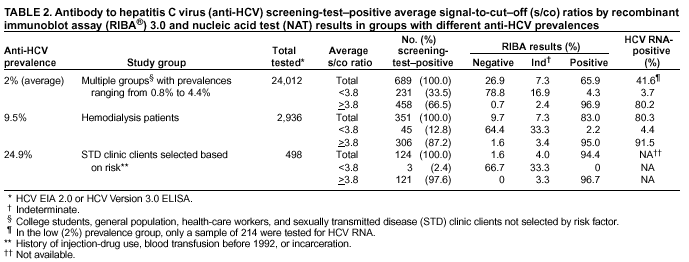
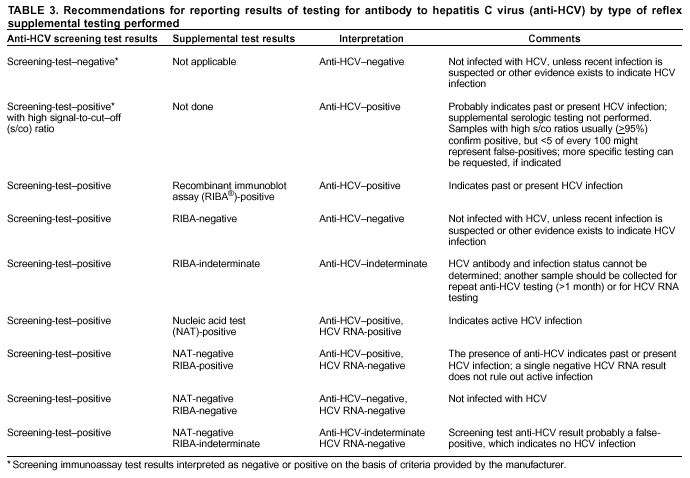
Guidelines For Laboratory Testing And Result Reporting Of Antibody To Hepatitis C Virus
Hcv Rna Pcr Qualitative Test Results Normal Range のギャラリー
Module 4 The Main Types Of Test Relevant To The Diagnosis And Management Of Hcv

Systematic Reviews And Evidence Summaries Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf
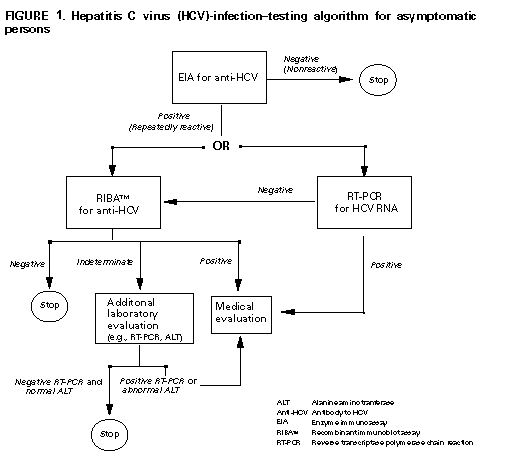
Hepatitis C In Pregnancy
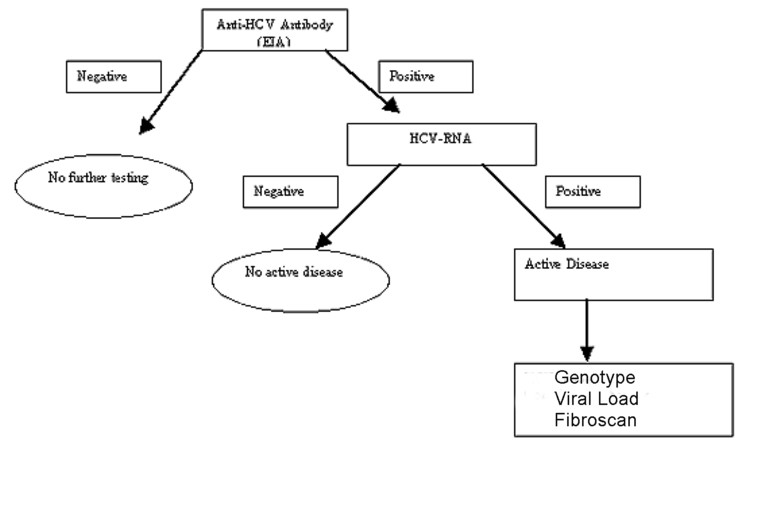
Hep C Tests Results Information Explanation And Costs

Evaluation Of Five Screening Tests Licensed In Argentina For Detection Of Hepatitis C Virus Antibodies

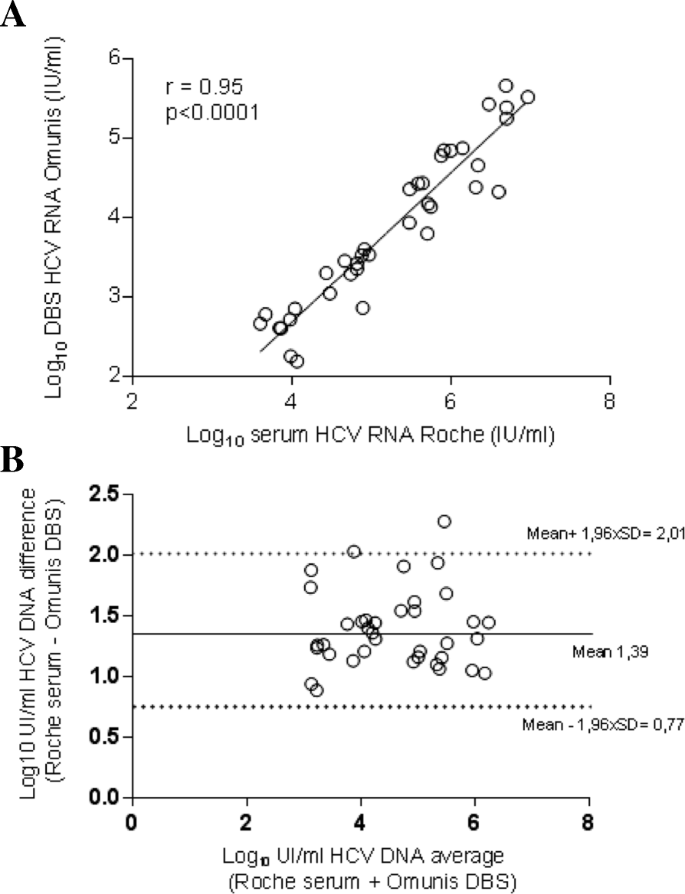
Swiss Medical Weekly Rapid Point Of Care Hcv Rna Quantification In Capillary Whole Blood For Diagnosing Chronic Hcv Infection Monitoring Treatment And Detecting Reinfection
Q Tbn 3aand9gcsbmffjrdiarqefgtt2g9t4kju6kxbihw8wwee8ha67nhb1qa8a Usqp Cau

Hcv Rna Pcr Test Qualitative And Quantitative Results Dieta Efectiva
Module 4 The Main Types Of Test Relevant To The Diagnosis And Management Of Hcv

Swiss Medical Weekly Rapid Point Of Care Hcv Rna Quantification In Capillary Whole Blood For Diagnosing Chronic Hcv Infection Monitoring Treatment And Detecting Reinfection

Utility Of Routine Real Time Quantitative Pcr Monitoring Of Hcv Infection In Haemodialysis Patients Datta S Goel N Wattal C Indian J Med Microbiol
Www Hepatitisc Uw Edu Pdf Treatment Infection Monitoring Core Concept All

Swiss Medical Weekly Rapid Point Of Care Hcv Rna Quantification In Capillary Whole Blood For Diagnosing Chronic Hcv Infection Monitoring Treatment And Detecting Reinfection

Positive Test Results Obtained By The Three Quantitative Hcv Rna Assays Download Table

Primary Screening Of Blood Donors By Nat Testing For Hcv Rna Development Of An In House Method And Results

Quantitation Of Hcv Rna In Liver Of Patients With Chronic Hepatitis C

Comparison Between Patients With Or Without Intrahepatic Hepatitis C Download Table
Core Concepts Diagnosis Of Acute Hcv Infection Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online
Core Concepts Diagnosis Of Acute Hcv Infection Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online
Guidelines For Laboratory Testing And Result Reporting Of Antibody To Hepatitis C Virus
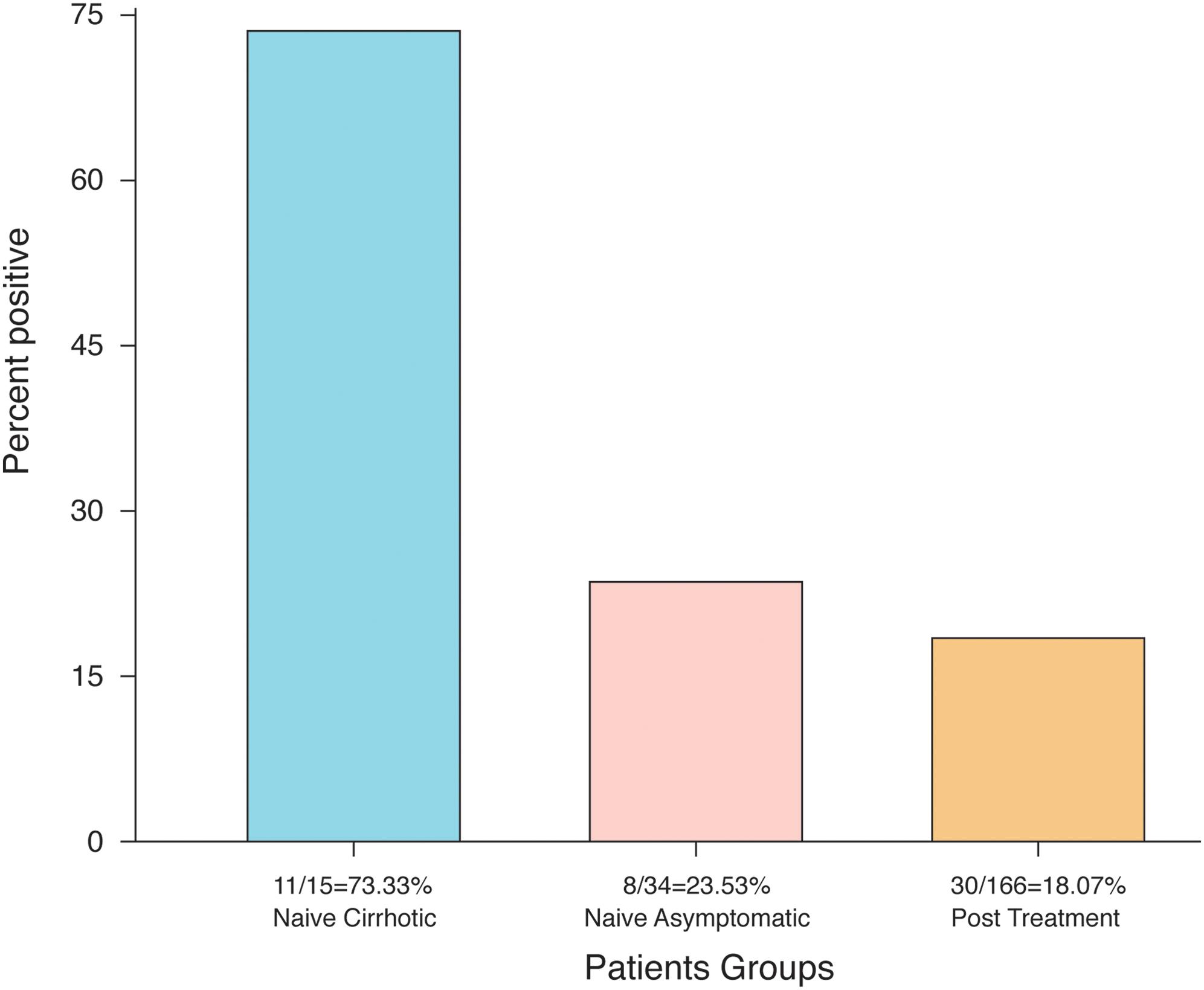
Hepatitis C Virus Rna Strands Detection In Peripheral Blood Mononuclear Cells Legitimizes Virus Eradication In Negative Serum Pcr Naive And Post Treatment Patients
Guidelines For Laboratory Testing And Result Reporting Of Antibody To Hepatitis C Virus

Modeling Of Patient Virus Titers Suggests That Availability Of A Vaccine Could Reduce Hepatitis C Virus Transmission Among Injecting Drug Users Science Translational Medicine

A Complete Molecular Biology Assay For Hepatitis C Virus Detection Quantification And Genotyping

Hepatitis C Virus Detection In The Semen Of Infected Patients

Range Of Hcv Rna Titers According To Histologic Activity Index Hai By Download Table

Jrp Decentralized Community Based Hepatitis C Point Of Care Testing And Direct Acting Antiviral Treatment For People Who Inject Drugs And The General Population In Myanmar Protocol For A Feasibility Study Draper Jmir

Serum And Liver Hcv Rna Levels In Patients With Chronic Hepatitis C Correlation With Clinical And Histological Features Gut

Table 1 From Evaluation Of Performances Of Versant Hcv Rna 1 0 Assay Kpcr And Roche Cobas Ampliprep Cobas Taqman Hcv Test V2 0 At Low Level Viremia Semantic Scholar

Linearity Panels Hiv Rna Hcv Rna Hbv Dna And Cmv Dna Linearity Panel Design

Utility Of Routine Real Time Quantitative Pcr Monitoring Of Hcv Infection In Haemodialysis Patients Datta S Goel N Wattal C Indian J Med Microbiol
Confirmation Of Hcv Viremia Using Hcv Rna And Core Antigen Testing On Dried Blood Spot In Hiv Infected Peoples Who Inject Drugs In Vietnam Bmc Infectious Diseases Full Text
Laboratory Diagnostics In Hepatitis Ppt Video Online Download
Core Concepts Hepatitis C Diagnostic Testing Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online

Hepatitis C Virus Core Antigen Assay Can We Think Beyond Convention In Resource Limited Settings

Pdf Performance Characteristics Of A Real Time Rt Pcr Assay For Quantification Of Hepatitis C Virus Rna In Patients With Genotype 1 And 2 Infections
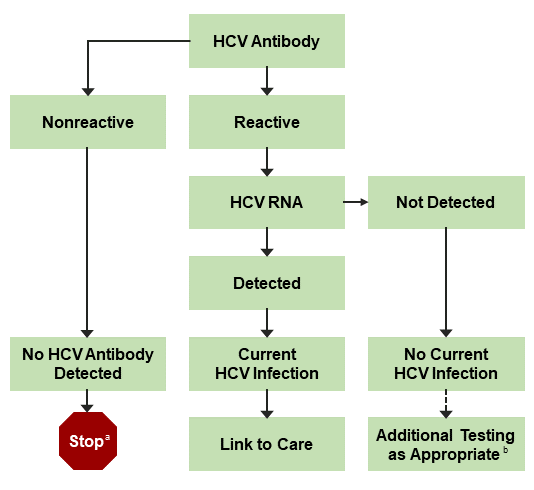
Hcv Testing And Linkage To Care Hcv Guidance
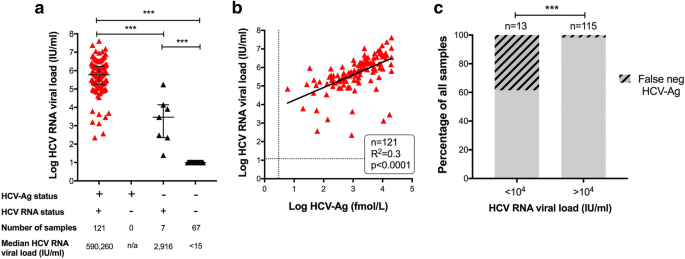
Hepatitis Virus Hcv Diagnosis And Access To Treatment In A Uk Cohort Bmc Infectious Diseases Full Text

Qualitative Detection Of Hepatitis C Virus Rna Comparison Of Analytical Sensitivity Clinical Performance And Workflow Of The Cobas Amplicor Hcv Test Version 2 0 And The Hcv Rna Transcription Mediated Amplification Qualitative Assay
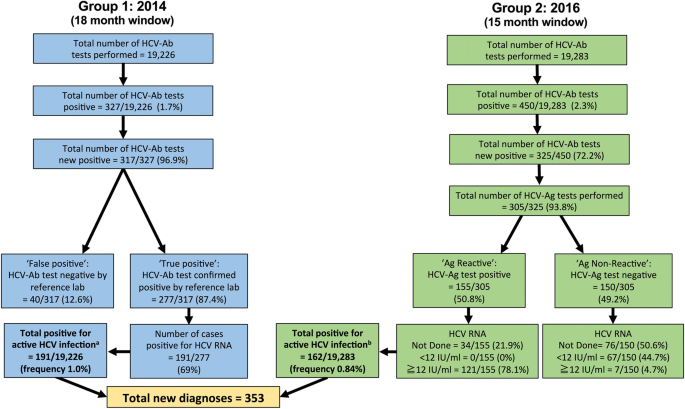
Hepatitis Virus Hcv Diagnosis And Access To Treatment In A Uk Cohort Bmc Infectious Diseases Full Text

Significance Of Anti Hcv Signal To Cutoff Ratio In Predicting Hepatitis C Viremia

Primary Screening Of Blood Donors By Nat Testing For Hcv Rna Development Of An In House Method And Results
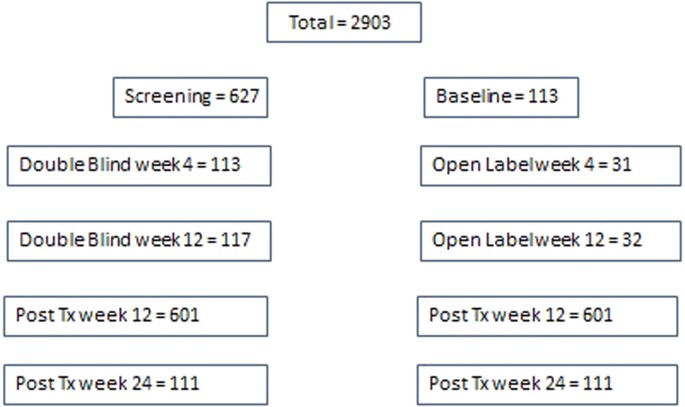
Hepatitis C Rna Assay Differences In Results Potential Implications For Shortened Therapy And Determination Of Sustained Virologic Response Scientific Reports

Hepatitis C Virus Hcv Diagnosis Epidemiology And Access To Treatment In A Uk Cohort Biorxiv
Q Tbn 3aand9gct7lehfgx2xlkxzmjkk33wcdkpdyijhyl6bn4kw7ne Usqp Cau
Interpreting Your Biopsy And Lab Results Ppt Video Online Download

Performance Evaluation Of The Versant Hcv Rna Qualitative Assay By Using Transcription Mediated Amplification Journal Of Clinical Microbiology

Hepatitis C Testing Hepatitis C Treatment
Hcv Core Antigen Is An Alternative Marker To Hcv Rna For Evaluating Active Hcv Infection Implications For Improved Diagnostic Option In An Era Of Affordable Daas Peerj

Treatment Of Acute Hepatitis C With Interferon Alfa 2b Nejm

Full Text Detection Of Hepatitis C Virus Hcv Among Health Care Providers In An Idr
400 000 Iu Ml Is New Cut Off For Low Vs High Viral Load In Hcv

Overestimation And Underestimation Of Hepatitis C Virus Rna Levels In A Widely Used Real Time Polymerase Chain Reaction Based Method Chevaliez 07 Hepatology Wiley Online Library

Hepatitis C Part I Routine Serologic Testing And Diagnosis American Family Physician

A Highly Specific And Sensitive Hepatitis C Virus Antigen Enzyme Immunoassay For One Step Diagnosis Of Viremic Hepatitis C Virus Infection Hu 16 Hepatology Wiley Online Library

Hepatitis C Virus Core Antigen A Simplified Treatment Monitoring Tool Including For Post Treatment Relapse Sciencedirect
An Update On The Management Of Hepatitis C Guidelines For Protease Inhibitor Based Triple Therapy From The Latin American Association For The Study Of The Liver Annals Of Hepatology
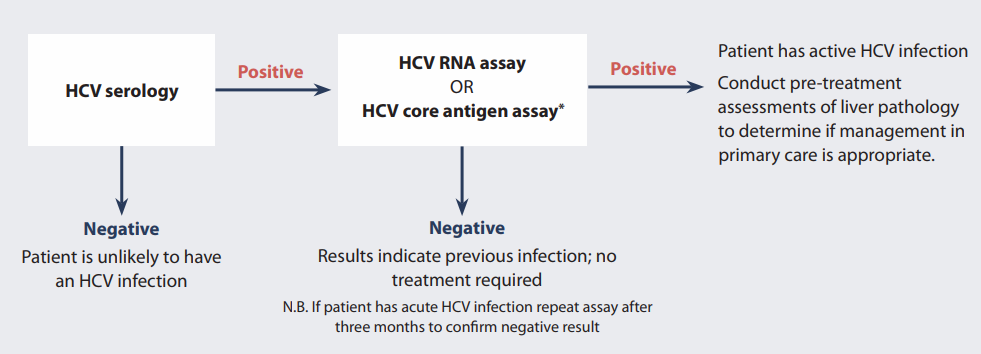
Testing For Hepatitis C Virus Hcv In Patients At High Risk Of Infection Bpacnz
Q Tbn 3aand9gcq9hzwv 5daugpz 7fz7 S32sxyxm Psjmswpdx0gq9o0aqkdrh Usqp Cau

Systematic Reviews And Evidence Summaries Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf

Commercially Available Quantitative Real Time Pcr Based Hepatitis C Download Table

Pdf Multicenter Quality Control For The Detection Of Hepatitis C Virus Rna In Seminal Plasma Specimens
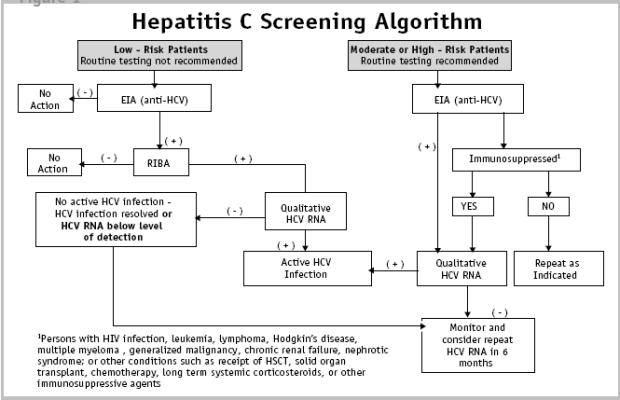
Clinical Guidelines For The Medical Management Of Hepatitis C
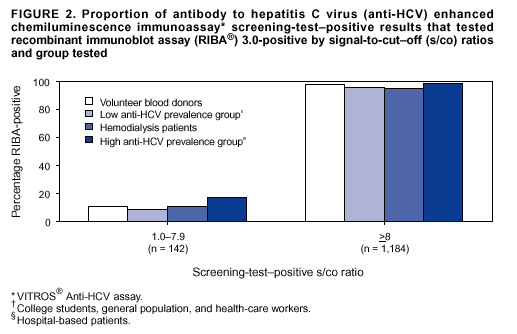
Guidelines For Laboratory Testing And Result Reporting Of Antibody To Hepatitis C Virus
Www Who Int Hepatitis Publications Annex 5 7 Pdf Ua 1
Www Who Int Hepatitis Publications Annex 5 6 Pdf Ua 1

The Warde Report Issue 27 2 New Testing Options For Hepatitis C Virus

Core Concepts Hepatitis C Diagnostic Testing Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online

Jpma Journal Of Pakistan Medical Association
Hcv Rna Pcr How This Hepatitis C Virus Test Works Results More

Pdf Evaluation Of Performances Of Versant Hcv Rna 1 0 Assay Kpcr And Roche Cobas Ampliprep Cobas Taqman Hcv Test V2 0 At Low Level Viremia
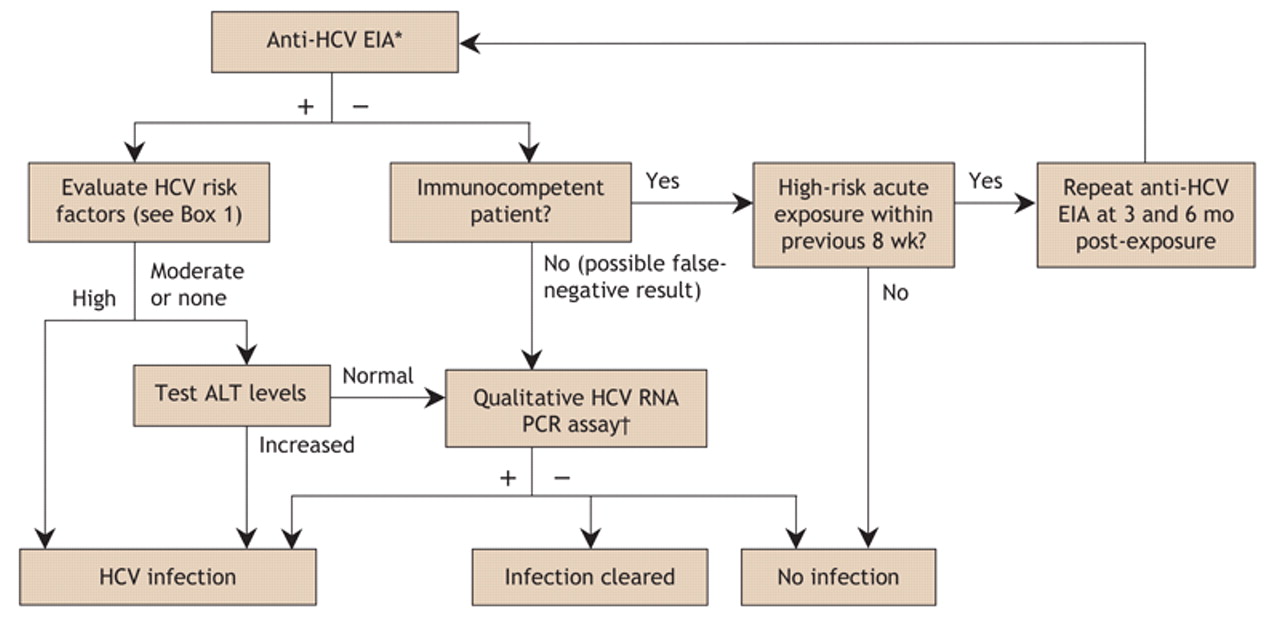
Hepatitis C A Review For Primary Care Physicians Cmaj

Transmission Of Hepatitis C Virus From Mothers To Infants Nejm

Qualitative Rt Pcr Results Obtained By 12 Laboratories For Detection Of Download Table
Hepatitis C Antibody Test Results And What To Expect

Swiss Medical Weekly Rapid Point Of Care Hcv Rna Quantification In Capillary Whole Blood For Diagnosing Chronic Hcv Infection Monitoring Treatment And Detecting Reinfection
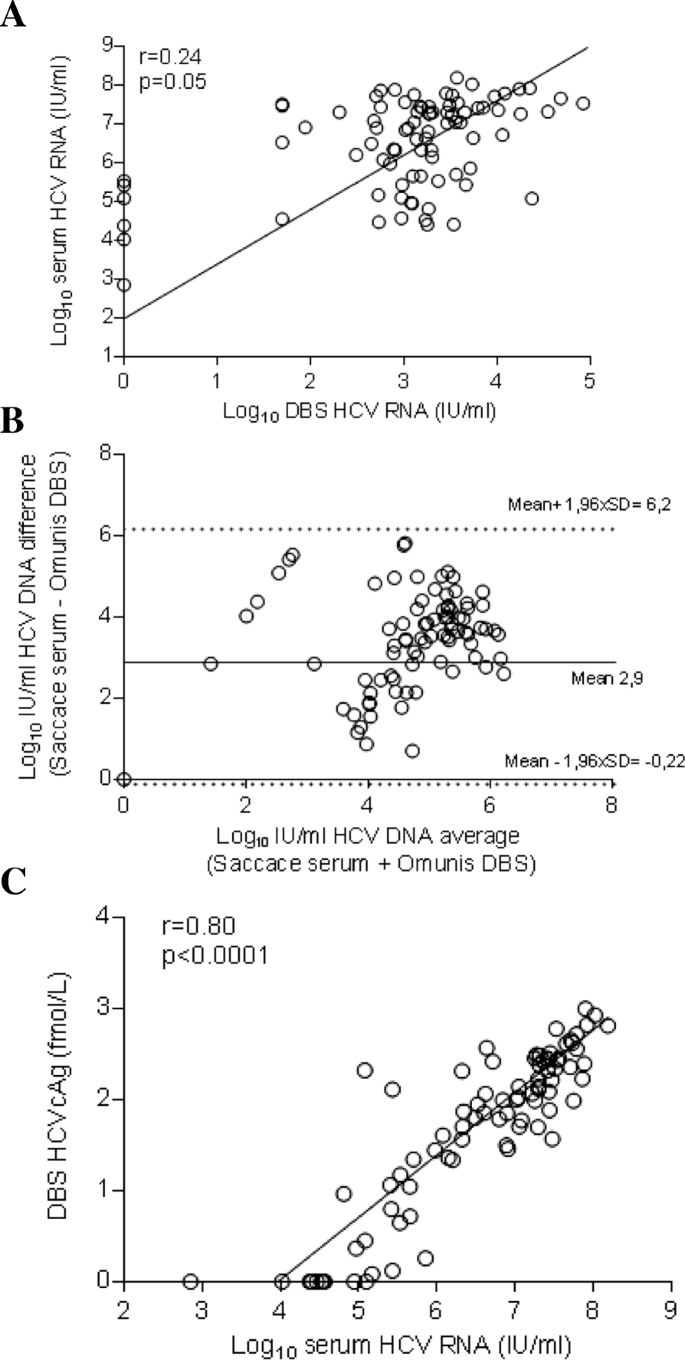
Confirmation Of Hcv Viremia Using Hcv Rna And Core Antigen Testing On Dried Blood Spot In Hiv Infected Peoples Who Inject Drugs In Vietnam Bmc Infectious Diseases Full Text
Ultrasensitive Hcv Rna Quantification In Antiviral Triple Therapy New Insight On Viral Clearance Dynamics And Treatment Outcome Predictors

Hcv

Evaluation Of The Aptima Hcv Quant Dx Assay Using Serum And Dried Blood Spots Journal Of Clinical Microbiology

Hcv Rna Pcr What To Know About Hepatitis C Testing
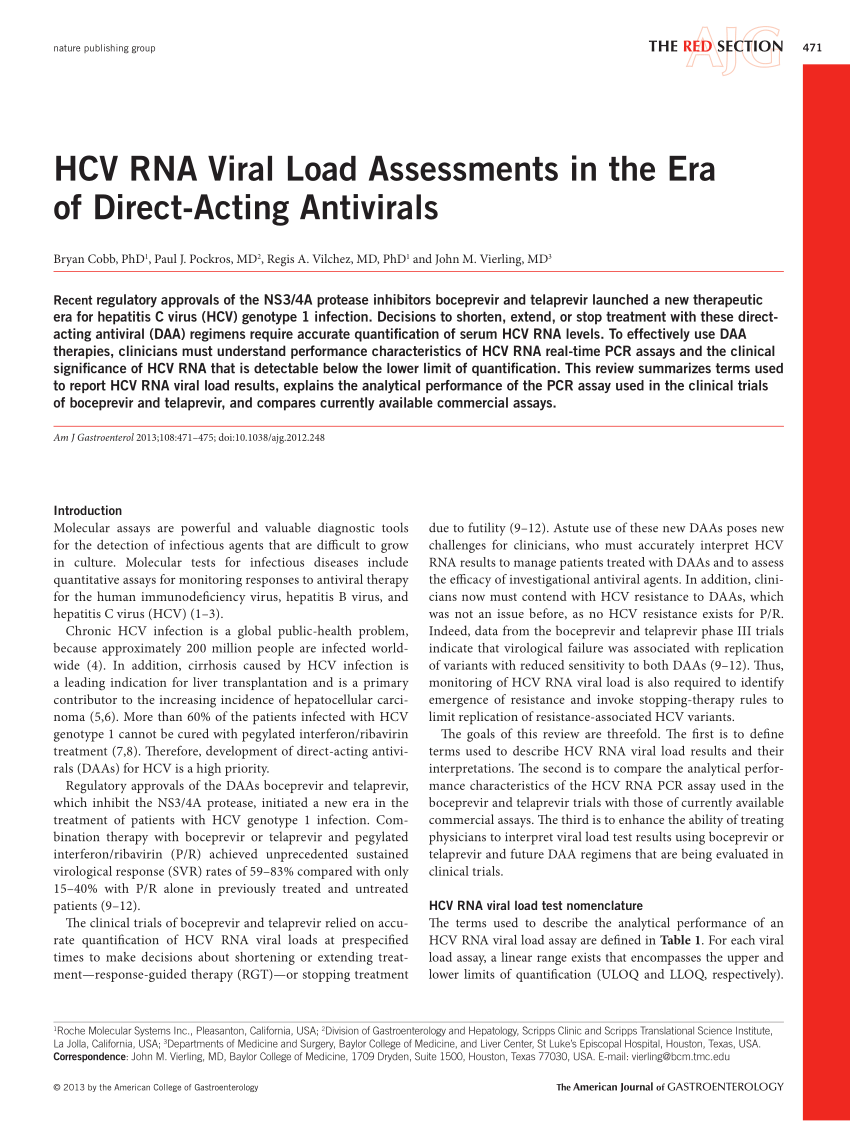
Pdf Hcv Rna Viral Load Assessments In The Era Of Direct Acting Antivirals

Hepatitis And Interferons

Full Text Detection Of Hepatitis C Virus Hcv Among Health Care Providers In An Idr
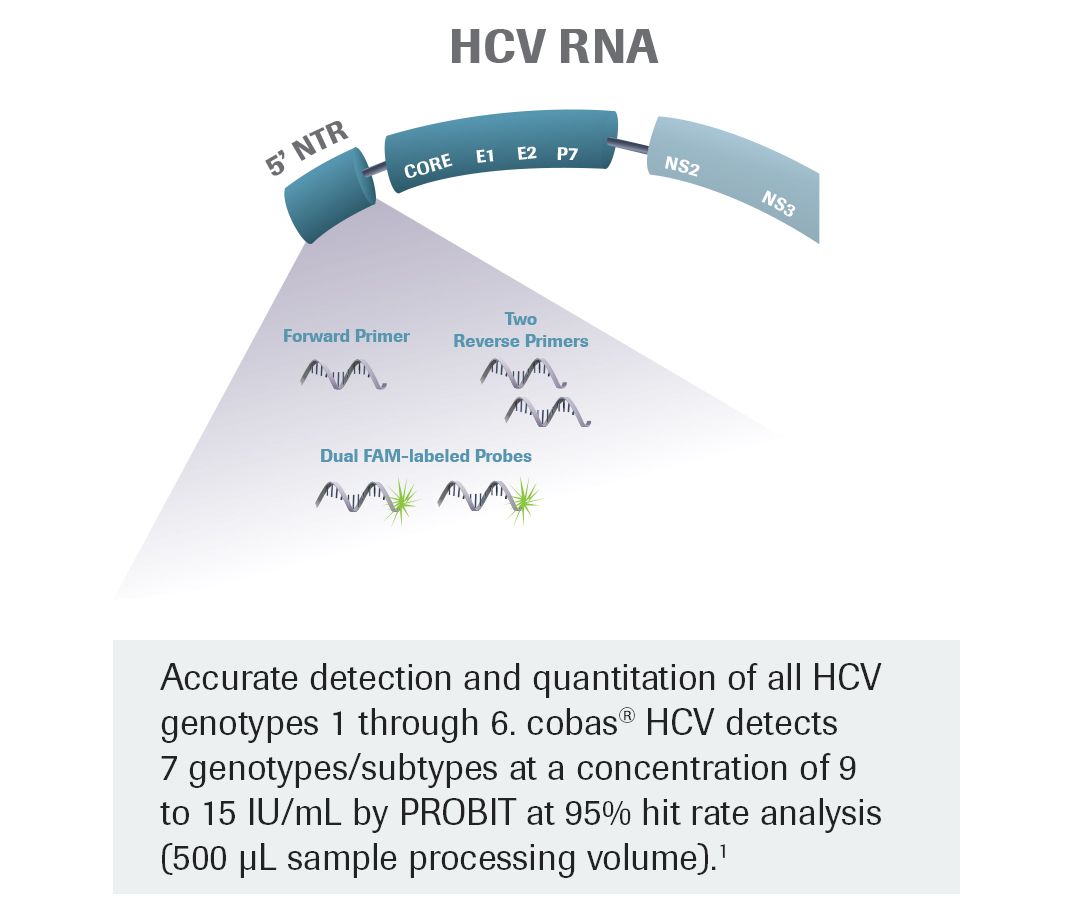
Cobas Hcv Test

A Rational Use Of Laboratory Tests In The Diagnosis And Management Of Hepatitis C Virus Infection Sciencedirect
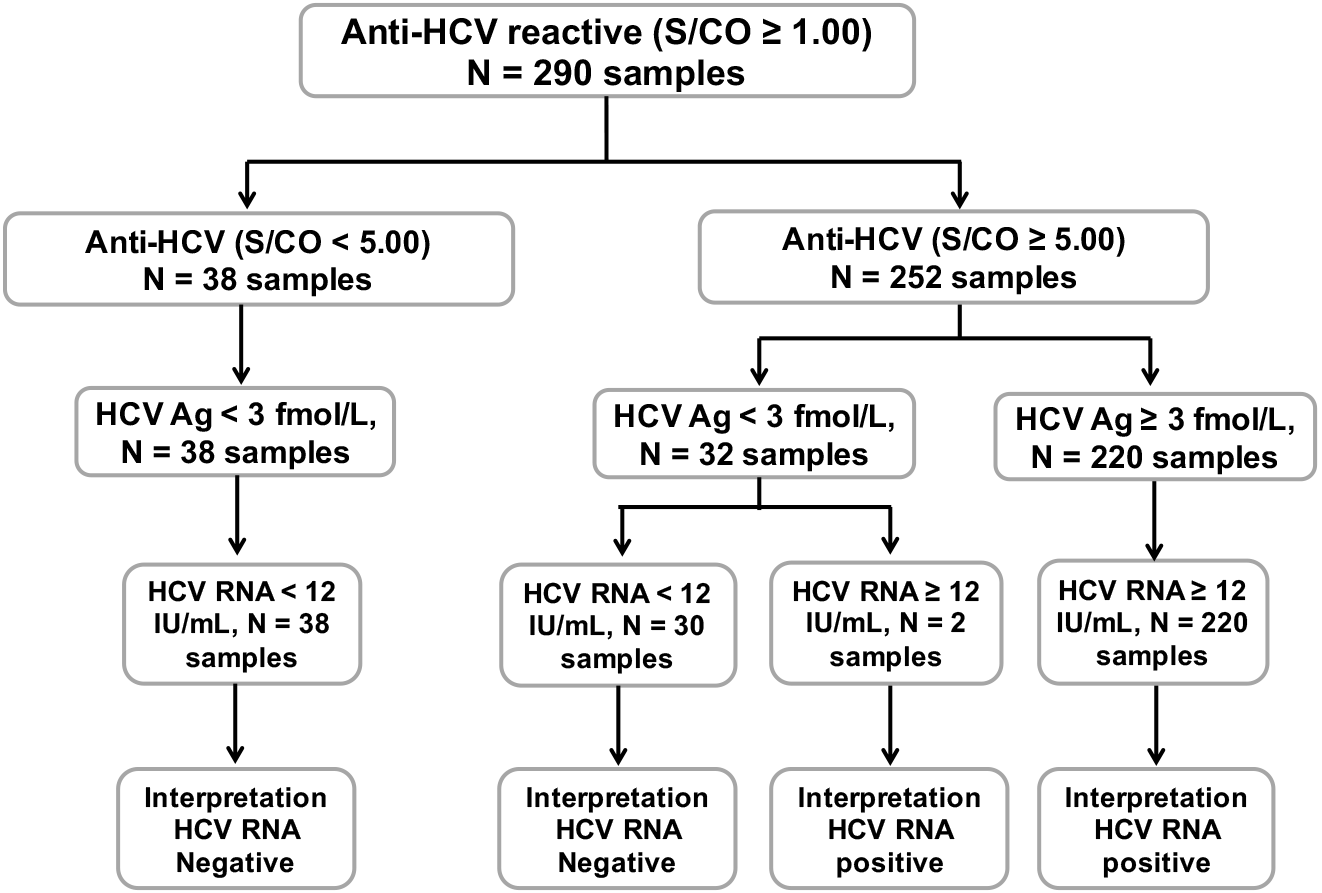
Hcv Core Antigen Is An Alternative Marker To Hcv Rna For Evaluating Active Hcv Infection Implications For Improved Diagnostic Option In An Era Of Affordable Daas Peerj
A Novel Multiplex Real Time Pcr Assay For The Concurrent Detection Of Hepatitis A B And C Viruses In Patients With Acute Hepatitis
Signs And Symptoms Of Hepatitis C Virus Infection Page 4 Of 5 Clinical Advisor

Evaluation Of The Cobas Hepatitis C Virus Hcv Taqman Analyte Specific Reagent Assay And Comparison To The Cobas Amplicor Hcv Monitor V2 0 And Versant Hcv na 3 0 Assays Journal Of Clinical Microbiology
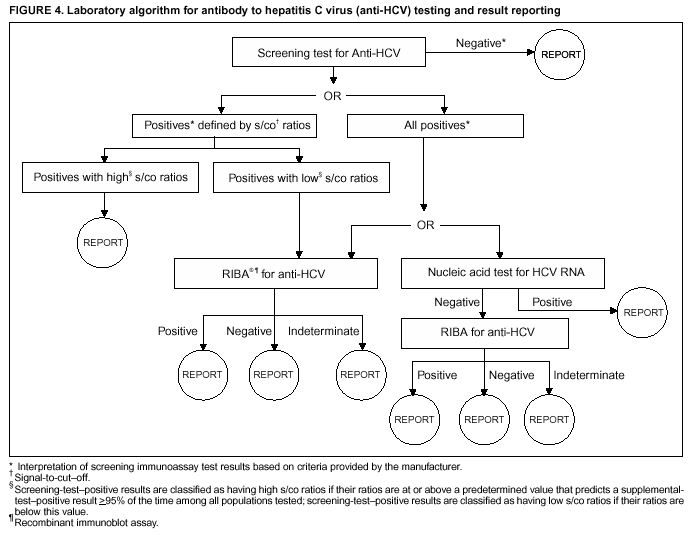
Guidelines For Laboratory Testing And Result Reporting Of Antibody To Hepatitis C Virus

A Clinical Epidemological Laboratorial Histological And Ultrasonographical Evaluation Of Anti Hcv Eia 2 Positive Blood Donors

Swiss Medical Weekly Rapid Point Of Care Hcv Rna Quantification In Capillary Whole Blood For Diagnosing Chronic Hcv Infection Monitoring Treatment And Detecting Reinfection

Signal To Cut Off S Co Ratio And Detection Of Hcv Genotype 1 By Real Time Pcr One Step Method Is There Any Direct Relationship
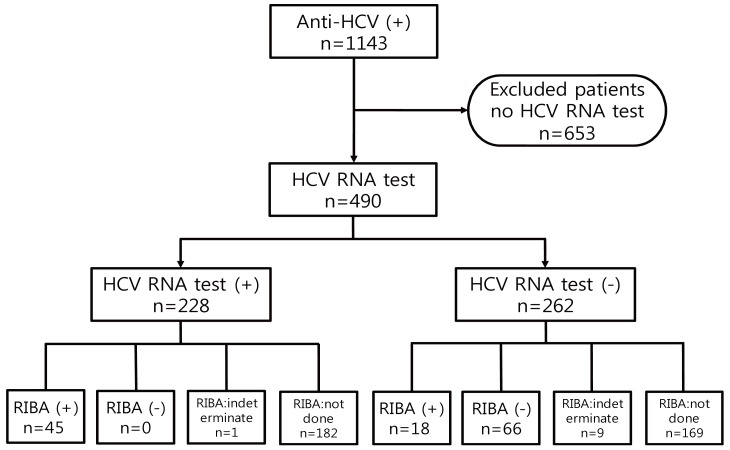
Hcv Rna Test

Diagnostic Tests And Test Results In Suspected Hcv Infection Download Table
Q Tbn 3aand9gcsf2 Pox2upnotm56whrd0khgrimaadlykabtxj Pdazc607ytg Usqp Cau
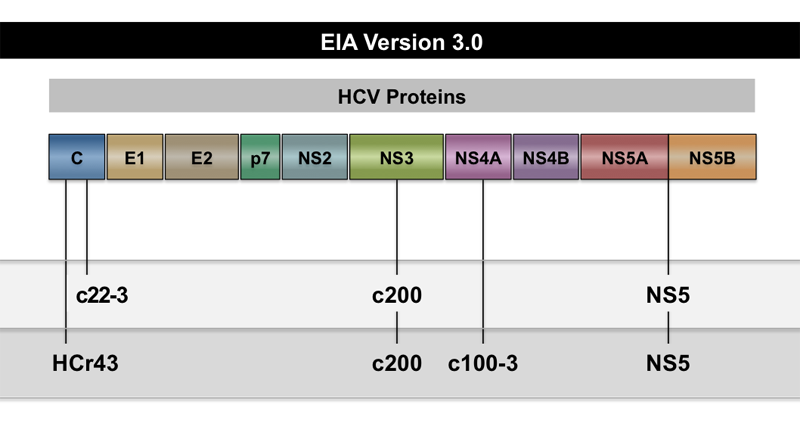
Core Concepts Hepatitis C Diagnostic Testing Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online

Improved Version 2 0 Qualitative And Quantitative Amplicor Reverse Transcription Pcr Tests For Hepatitis C Virus Rna Calibration To International Units Enhanced Genotype Reactivity And Performance Characteristics Journal Of Clinical Microbiology
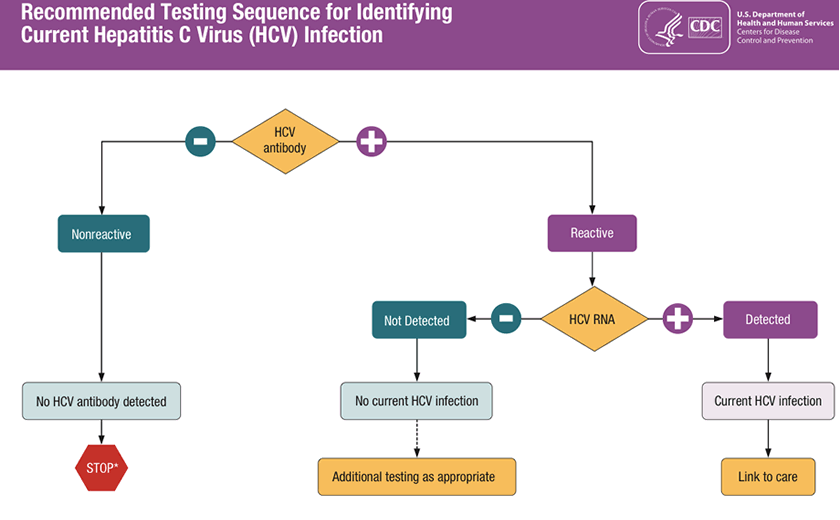
Untitled Document

Evaluation Of Assay Methods And False Positive Results In The Laboratory Diagnosis Of Hepatitis C Virus Infection Insight Medical Publishing



